Frequently Asked Questions
Why do we need a testable hypothesis?
We need a solution (mechanism of operation) for this system that has nearly 1011 neurons that are connected through 1015 synapses. The system is showing large number of disparate features that cannot be easily inter-connected. Moreover, its most important property is generation of internal sensations of various higher brain functions such as perception, memory and consciousness. So, now we need to find a solution that can unify all the disparate features. We need to arrive at a hypothesis that can provide testable predictions. According to Karl Popper, a philosopher of science, a hypothesis must be falsifiable; i.e. it must at least in principle be possible to make an observation that would disprove the proposition as false, even if one has not actually (yet) made that observation (Popper, 1965). A single counter example of proof against a hypothesis can then be used as sufficient reason to modify or reject it. Once such an observation is made, it will lead to the rejection of the hypothesis. However, even with rejection, we are likely to make some conclusions that will aid in the development of new and better testable hypotheses.
The nervous system is being studied by several faculties of sciences at various levels – biochemical, cellular, electrophysiological, systems, behavioural, imaging. In order to explain all these features, the solution must be a unique one. In this regard, a testable hypothesis is highly valuable. Even though the internal sensations cannot be directly examined, we can circumvent the difficulty. If a simple unique solution can be derived to explain all the findings made at various levels, then this solution must be right (This is similar to viewing an unknown variable in an equation within a solvable system of linear equations where the values of all the other variables are known. Please see this example). This motivated to develop a hypothesis for nervous system functions. The hypothesis can then be verified in biological systems by a) postdictive examination of several previous findings to test whether they can be explained by the hypothesized mechanism, b) searching for the predictions that can be made from the hypothesis, and b) examining comparable circuit features for different sensations in remote species of animals. Once verified, it can be further studied by the gold standard test of replicating the mechanism in engineered systems. This approach will truly enable us to undertake a cost-effective research work in the right direction.
Why should we study the formation of first-person inner sensations? Can't we understand the brain without studying it?
Each organ in the body is formed to execute very specific functions. For example, heart is formed to pump blood to other organs. Each and every cell in the heart is associated with this function, one way or other. We can't think of studying the heart by ignoring its pumping action. Similarly we can't think of studying the kidneys by ignoring their filtering action. If we look at the brain, its most important function is generation of first-person inner sensations of different brain functions such as perception, memory, and thought process. It also executes motor actions based on the survival needs determined by the first-person inner sensations of decision making. Due to this reason, we can't study the brain by ignoring its unique & most important function of generation of inner sensations that we call "mind." Wiring of the brain is evolved to generate robust first-person inner sensations within it, which is essential for the very survival of all the animals. We have not been paying attention to the probable locations and mechanism by which it is generated, most likely due to our lack of confidence in discovering methods to verify them. When we ignore pathways that generate first-person inner sensations, we are ignoring the major circuit connections that are responsible for their generation.
Why didn't we show interest to understand the mechanism that generates first-person properties?
Third person experimenters don't have tools to directly study first-person inner sensations. This stands as a brick wall against any simple approaches. Then, the system exhibits very large number of features that are being studied by different branches of brain science. To bring constraints from all of these findings and put them together is a gigantic task. All these factors generates an impending fear of failure among individual researchers. Then, we haven't put together a consortium of scientists from different fields towards this goal yet.
What function should we begin examining to build the hypothesis?
Learning and memory are the best functions to study the nervous system operations. This is because we can 1) induce changes in the nervous system during associative learning that can be verified, 2) induce first-person internal sensation of retrieved memories in physiological time-scales, 3) carry out loss of function studies, 4) test whether the hypothesis can be extended to understand consolidation of memories, perception and consciousness, 5) replicate in engineered systems to test for the formation of the first-person inner sensation of memory, and 6) use the very large amount of already collected data to verify the hypothesis being built at its various stages. For example, the following questions can be addressed. a) What parallel cellular changes are taking place during testing for long-term potentiation (LTP) with a regular stimulus and retrieval of memories? b) How LTP can get correlated with the surrogate markers of behavioural motor activities indicative of the induction of internal sensation of memory?
How does artificial intelligence community see first-person properties recently?
Members of AI community have started wondering how neuroscientists approach towards providing a mechanistic explanation for cognition. They want neuroscience to figure out the mechanism by which brain generates its functions. For example read: Neurotechnology is Critical for AI Alignment (cvitkovic.net)
What is the difference between single synapse strengthening hypothesis and semblance hypothesis?
From the Hebb’s postulates, it was derived that synaptic plasticity changes the strengths of single synapses during learning. According to this postulate, if two stimuli are associatively learned, then the synapses along their paths are expected to undergo plasticity changes. However, it is not yet known how the arrival of one of the stimuli (cue stimulus) that propagates through its path utilizes the changes in synaptic strength to induce memory of the associatively learned second item. All the studies of synaptic plasticity changes occurring at the time of learning rely on animal behavior at the time of memory retrieval. It is difficult to interconnect synaptic plasticity changes with behavior and derive a mechanistic explanation for memory. In other words, until now it was not possible to find an explanation that will allow us to replicate the postulated mechanism in an engineered system. It is important to note that Hebb's postulates have guided our research until now, which has provided a very large number of observations. However, difficulties in obtaining a mechanistic explanation for the first-person internal sensation of memory from learning-induced changes that can be replicated in engineered systems prompt us to re-examine the Hebb's postulates, identify its drawbacks and formulate a new postulate. In this context, the present hypothesis was developed by asking the question, "At the time of memory retrieval, when one of the sensory stimuli (the cue stimulus) propagates through its path, how can it induce an inner sensation of memory of the associatively learned sensory stimulus (that moved through a second path at the time of learning) and also generate behavioral motor activity reminiscent of the associatively learned second stimulus?"
Based on the semblance hypothesis, when an associative learning takes place between two sensory stimuli, there should be certain changes at the locations where these stimuli converge (for example, hippocampus in spatial memory or amygdala in fear memory). This hypothesis examined the interaction between the synapses of the associatively learned stimuli at locations of their convergence. At a later time, when one of the stimuli (cue stimulus) arrives at the locations of convergence of the two sensory stimuli, the cue stimulus should be able to induce internal sensation of the memory of the associatively learned second stimulus within the physiological time-scales of milliseconds. Therefore, semblance hypothesis focused on identifying the locus of interaction between the two neuronal pathways and more specifically, the sub-synaptic locations that belong to these two pathways between which learning induces certain changes from which the cue stimulus can induce inner sensations of memory of the second stimulus. In this approach, it is not possible to use neuronal firing due to several reasons explained in the answer to the next question.
What are the limitations of studying neuronal firing (somatic spike) in understanding higher brain functions?
Studies using behavior as a surrogate marker for memory have detected firing of specific sets of neurons during both learning and memory retrieval. For example, compared to the set of neurons that fire when exposed to one of the associatively learned stimuli (cue stimulus) before learning, additional neurons are fired when an animal is exposed to the same cue stimulus after learning. This is documented in the lateral amygdala in fear conditioning experiments (Schoenbaum et al., 1998; Tye et al., 2008). Manipulation of neuronal firing also resulted in identification of firing of specific sets of neurons during learning & memory retrieval (Tonegawa et al., 2015; Josselyn and Tonegawa, 2020). However, neural network studies being carried out for more than fifty years are finding severe difficulties in solving the nervous system. When we find a replicable mechanism of learning that can be used to generate memories, it is hoped that we will be able to explain how ensembles of neurons fire during learning and memory retrieval. In the light of necessity to understand memories in their true nature as first-person inner sensations, examination of conditions under which a neuron fire shows the following findings that need urgent consideration.
1) Investigations during the last 15 years have shown that in addition to axonal spikes (neuronal firing or action potential), there are spiking potentials occurring at the dendrites (Antic et al., 2010; Moore et al., 2017). Spikes are the instantaneous summation (summing up) of potentials occurring in a localized region. The purpose of the axonal spike is to propagate the potentials towards all the axonal terminals of the neurons. However, we have to still discover the function of the dendritic spikes. Only by directing our studies to interconnect as many observations as possible, we will be able to find the functional attributes of dendritic spikes that will help us to solve the system.
2) The number of input connections (postsynaptic terminals or postsynapses or dendritic spines) varies widely among the neurons. It ranges from one (passive conductance of potentials between the initial orders of neurons of the visual pathway) to approximately 5,600 (as in a monkey’s visual cortex) to 60,000 (as in a monkey’s motor cortex) (Cragg 1967). Most often, the arrival of a tiny fraction of inputs is sufficient to fire a neuron. Several earlier experiments provided hints that spatial summation of nearly 40 inputs arriving at neuronal soma can generate an action potential (neuronal firing). Recent modelling studies have shown that a pyramidal neuron that has tens of thousands of input connections can fire an action potential by spatial summation (summation at the same time) of nearly 140 EPSPs at the axonal hillock that arrives from randomly located dendritic spines (Palmer et al. 2014; Eyal et al., 2018) (Note that it is possible to have nearly 40 to 50 EPSPs of high strength originating close to the soma that can fire a neuron. For further discussions, the number 140 will be used). Please note that temporal summation of even less than 140 EPSPs can induce an action potential. The combinatorial probability of the number of sets of synapses whose activation can give rise to the firing of a neuron is enormously high. This makes an action potential non-specific with regards to its inputs.
3) Thirdly, postsynaptic potentials contributing to both sub- and supra-threshold activation of a neuron do not contribute to the neuronal firing. Therefore, if there are mechanisms for inducing internal sensations occurring at the unaccounted synapses, they will get ignored if neuronal firing alone is examined. For example, let us take one pyramidal neuron (excitatory neuron) with 25,000 inputs (dendritic spines). If 3600 inputs (dendritic spines) are activated simultaneously (due to their synaptic activation) during an action, only one action potential will be elicited. A simultaneous arrival of 140 inputs at the axonal hillock is enough to induce that action potential. This means (3600 - 140) = 3460 EPSPs get wasted without having any functional use. Is this advantageous to the system? For the purpose of this discussion, let us assume that 140 EPSPs can fire a neuron. In this context, any set of inputs of less than 140 EPSPs that do not lead to the generation of the action potential is also getting wasted. In what context evolution would have conserved this mechanism? The input redundancy may be a possible mechanism to achieve a common set of outputs for operating the limited set of combinations of muscles in the body for achieving behavioral activities to survive in the environment. When a cue stimulus activity propagates to one of the inputs to a neuron at its sub- or supra-threshold activation state and if it does not lead to change in the non-firing/firing state of that neuron, can the information from the cue stimulus still be utilized by the system? In the context that we are still searching for a mechanism of induction of first-person internal sensation of memory, reminiscent of the sensory features of the learned item in its absence, it is necessary to examine a possible mechanism that occurs at the input level.
4) Postsynaptic potentials induced at the dendritic spines located at remote locations on the dendritic tree (for example, pyramidal neurons with long apical dendritic tree) have to travel long distances to reach towards the axon hillock to summate above the threshold for triggering the action potential. They degrade significantly as they reach the axon hillock (Spruston 2008). Therefore, the contributions of these potentials to neuronal firing get reduced and vary depending on the distance they have to travel and the dendritic diameter. This naturally leads to the question "Why would the observed degradation of potentials get conserved?" It is very likely that they are providing functions independent of the neuronal firing except in conditions where they contribute to the nth EPSP necessary to trigger an action potential. Whenever a postsynaptic potential degrades (attenuate), information is gradually lost. Therefore, we have to think about a mechanism other than neuronal firing. For preventing loss of information, it is necessary to have an operational mechanism taking place close to the origin of inputs (postsynaptic potentials), which will not be affected by the attenuation of postsynaptic potentials. This will provide an efficient mechanism whereby all the specific inputs can contribute to generate a specific brain function (e.g. specificity of memory).
5) Since EPSPs get degraded as the distance from the dendritic spine to the soma increases, in reality EPSPs from nearly 140 dendritic spines will get summated to fire a neuron. Let us assume that this pyramidal neuron has 10,000 dendritic spines (inputs or postsynaptic terminals). If EPSPs arriving from nearly 140 of its dendritic spines can fire that neuron, then nearly [1x104! ÷ (140! x (1x104! – 140!))] ≈ 2.79x10318 sets of combinations of input signals can fire that neuron. If we consider that a pyramidal neuron has only 3,000 dendritic spines, then the set of combinations will reduce to 1.72x10244 (To compare, note that the number of atoms in the observable Universe is only nearly 1082). Note that above calculations are done only for a fixed number of 140 input signals. When the number of input signals varies from 141 to 10,000 or 141 to 3000 respectively, each possibility needs separate calculations to find the number of possible combinations. Therefore, the sum of all the possible combinations will be a huge number. This means that a gigantic number of combinations of input signals can cause the same neuronal firing. Therefore, when we see a neuron firing (axonal spike) (in vivo, at physiological conditions), it is not produced by any specific set of inputs. Understanding the extreme degeneracy of sets of input signals in firing a neuron is of paramount importance in making correlations between the firing of specific neurons (both natural and artificial) and those higher brain functions having unique internal sensations.
6) Many times, several neurons are held at sub-threshold activation. It means that they will be receiving less than 140 postsynaptic potentials all the time, just short of few potentials for triggering an action potential (neuronal firing). Neurons located at higher orders than those that are firing in an oscillating fashion (reasons for these oscillating type of neuronal firing need explanation, especially the horizontal component of the oscillations – which are explained by the present hypothesis) are mostly held at a range of sub-threshold values. For example, 138 or 139 inputs arriving at higher order neurons will not lead to the firing of those neurons. These sub-threshold-activated neurons require only 1 or 2 input signals to cause their firing. Therefore, when we see these neurons firing, these neuronal firings have to be interpreted completely differently.
All the above findings show that studies using neuronal firing and networks of firing-neurons do not examine specific mechanisms that are likely to take place at the level of the inputs (dendritic spines). In addition, when it comes to the need for explaining the first-person internal sensations of higher brain functions, current studies examining the third-person observations are a dimension away (third-person v/s first-person) from where we need to reach.
So what does a neuronal firing mean with respect to its inputs? From the above paragraphs, we have seen examples of conditions in which a neuron held at its baseline state can get fired by either 3600 inputs or just 1 input. In what context evolution would have conserved this mechanism? It may be a possible mechanism to achieve a common set of outputs for operating the limited set of combinations of muscles in the body for achieving behavioral activities to survive in the environment. In the context that we are still searching for a mechanism of induction of first-person internal sensations, reminiscent of that are induced by the external stimuli (in the latter's absence), it is required to examine possible mechanisms occurring at the input level. In the context of input redundancy in firing a neuron, this will avoid ignoring any valuable operational mechanism occurring at the input level. This will allow us to address the question from the previous subtitle "Where is the ideal location for convergence to occur that will allow the cue stimulus to induce internal sensation of the associatively learned second stimulus?" without ignoring the specificity of inputs brought by the cue stimulus. It is reasonable to expect interactive changes occurring at the input levels of the neurons at locations of convergence of associatively learned stimuli. This is examined in the new hypothesis.
Inner sensations cannot be accessed by third-person observers. Then, how can we study them?
To study things that our sensory systems do not have any access, we need to use the principles of the methods used in physics to study particles and fields to which also we do not have any access. The basic principle is based on the deep principle used in mathematics when finding a solution to a system of linear equations having a unique solution. It is the constraints provided by the terms in the equations that guide towards solving the system. In this approach, we need to use all the equations within the system to find that solution. Similarly, by using constraints from all the findings from various levels of the nervous system (see Table 2 on the first page of this website), it is possible to derive a solution for the system that induce units of internal sensations and integrate/process them at physiological time-scales. Once such a solution can be derived, then several postdictive findings can be examined for the validity of the solution. Once this stage succeeds, then predictions can be made that can be verified. This is a standard procedure used by physics to make discoveries. A similar approach can be undertaken to understand the location and mechanism of generation of units of internal sensations. The present work has followed these steps towards understanding the operational mechanism.
How can the information from fMRI studies be used to understand the operational mechanism?
From the Table 2 on the Home page of this website, it can be seen that one of the requirements of the operating mechanism is that it should take place at physiological time scales. Since blood oxygenation level dependent (BOLD) signals initiate very slowly and take nearly 4 seconds to peak following a higher brain function or neural activity at the same location (Fig.2 in Monti et al., 2010; Figs.2-5 in Murayama et al., 2010), it does not provide information regarding the normal operational mechanism. However, when the actual mechanism of operation is known, it should be able to provide an explanation why oxygen is released at those locations following a time delay. In other words, the hypothesized mechanism should be able to accommodate a proper explanation for the BOLD signals.
Which higher brain function can be studied to explore the generation of first-person inner sensations taking place in matching time scales of milliseconds?
Among different brain functions, memory has the advantage that experiments can be carried out both to associatively teach the system to examine learning-induced changes and can examine they might be used for memory retrieval. Since no cellular changes are observed during memory retrieval, memory retrieval is likely to take place by a passive reactivation of a learning-induced change. First, it is necessary to re-define memories. Memories are first-person virtual internal sensations of an item (in the absence of that item) in response to a cue stimulus or occurring spontaneously. Memories were classified into working, short-term, and long-term based on the duration between learning and memory retrieval. Since qualia (virtual first-person internal sensations) of these retrieved memories are almost the same, it is reasonable to hypothesize that a) a cellular mechanism is taking place during learning, and b) reactivation of learning-induced change retained for different duration can explain different types of memories classified based on the time of their retrieval.
What are the current challenges in memory research and how can we overcome them?
Memories are virtual internal sensations at the time of memory retrieval. The behavioral motor activities observed along with it should be considered as surrogate markers indicative of memory retrieval. Strong correlation between the experimental finding of long term potentiation (LTP) and the surrogate behavioral motor activities at the time of memory retrieval have been observed. However, alone, LTP has certain limitations. LTP takes at least 20 to 30 seconds (Gustafsson and Wigström, 1990) and even more than a minute to reach it's peak level of induction, which does not match with the physiological time-scales of changes occurring during associative learning. LTP was reported as lacking sufficiency to be the mechanism of memory storage (Shors and Matzel, 1997; Martin et al., 2000; Piorazi and Mel, 2001). Furthermore, several reported correspondences of LTP temporal phases do not correspond with that of memory phases (Abbas et al., 2015). In spite of these, the correlation between the behavioral markers of memory with LTP (excluding the time-scale issues) has some hidden facts that can provide a valuable piece of the puzzle towards understanding the cellular changes occurring during associative learning. In this context, it becomes necessary that the true mechanism of formation of first-person internal sensation of retrieved memories should be able to explain how LTP is related to memory.
Challenges in understanding the mechanistic changes during associative learning that enables cue-induced internal sensation of retrieved memory and its related effects on the observations in the field of psychology have been discussed (Gallistel and Balsam, 2014; Edelman, 2012). The challenges become manageable when it become possible to figure out a method to enter into the first-person frame of reference using third-person observed findings.
What are the general requirements for a hypothesis of memory?
It should be able to explain all the features of the system observed in its different levels. The solution offered by the hypothesis should be able to satisfy constraints offered by all the findings. See Table 2 in the Home page of this website for a list. Since studying learning and memory is an ideal one, it is necessary to have a mechanistic view of memories. Since sensation of a stimulus in its absence is hallucination, memories can be viewed as cue-induced cue-specific hallucinations (Minsky, 1980). Can we search for a learning-mechanism that can allow induction of virtual first-person internal sensations of memory as a cue-induced hallucination? This is the basis of developing the semblance hypothesis which was first published as a book in 2007 (book copy). Revised editions were published in 2008 and 2010. To become a valid scientific hypothesis, it must provide testable predictions that can be verified.
How much time does it take for the learning mechanism to occur?
Humans have an ability to associate more than one pair of sensory stimuli in a rapid-fire exercise during a limited period of one second. The learning changes induced by more than one pair of associative learning stimuli can then be used to retrieve their corresponding memories. This indicates that learning mechanism can be "completed" in sub-second (milliseconds) duration. Depending on the duration for which learning-indued changes persist, memory can be retrieved at different duration following learning. More details in a Preprint.
What is the most important step to succeed?
Associative learning induces changes in milliseconds (physiological time-scale). Using these changes (following learning), a cue stimulus can retrieve first-person internal sensation of memory in milliseconds. If the changes induced during the milliseconds of time during learning can remain within the system, then it should be able to provide the ability to retrieve memory after a long period of time, which we call as long-term memory. In this context, we must focus on changes occurring within millisecond time-scales at the time of learning. This should be the focus on understanding the science behind the operational functions of the nervous system. All the delayed molecular changes following learning can only have secondary effects on the primary change occurring during the milliseconds of time during learning.
How can a set of constraints guide towards the solution?
How can we reach the solution using a large number of constraints provided by findings from different levels of nervous system functions? This approach is motivated by the methods used in physics to understand particles and fields that we cannot sense directly using our sensory systems. The deep underlying principle of this is based on methods used in linear algebra for solving a system of large set of linear equations that has a unique solution. Here, the relationships between the variables in the equations guide us towards the solution. In mathematics, it is possible to find quick methods to arrive at the solution. In fact, we invent those quick methods. The natural question at this point is that mathematics can develop the equations. Neuroscience is different. Yes, in mathematics all the derivations can be carried out even without any equations. Equations were invented by us so that others can derive the results of similar problems very quickly. Always the first person who invent such short cuts need to spend lots of time to design it (In fact, students who study only the equations do not understand the concept behind the process and they will not like mathematics. Once one understands the process behind an equation, one will enjoy it and most likely go for graduate studies in mathematics!). So the point here is that if we are ready to spend time and energy, we can slowly arrive at the solution for the nervous system using the deep principle behind solving a system of linear equations.
Since in neuroscience, we cannot have such equations or shortcuts, we have to arrive at the solution using the hard way. Since we cannot create an easy equation in neuroscience, everyone who tries to understand the derived solution has to take the same hard way to appreciate the solution. Since studies of the brain has specialized and super-specialized into a large number of levels, those who are interested in understanding how the solution was derived will have to spend time to understand the different fields of these specializations. This is a reality.
Here we will use subsets of disparate findings from the list in Table 2 on the Home page of this website. We need to use trial and error methods to reach at the solution. By repeating this approach using different subsets of findings, we are expected to arrive at the same solution, which is expected to be the correct solution. Why do we have this much optimism? The optimism is due to the fact that there can only be one unique solution for the system and since we are using very large numbers of findings from different levels of operation of the system, it must be correct. At this point one may ask the following questions. “What is the problem with already published work in neuroscience?” Research work in neuroscience has been carried out by examining finding only from few levels to reach a solution. This has been the practice since one person can only specialize in a few levels of studies and journals have space limitations for articles. “What is the problem with already published work in neuroscience that explains synaptic plasticity?” Here, we made an assumption that synaptic connections make changes and it will be responsible for learning-induced changes from which memories are retrieved. This was initially set up not based on any derivations. Now that we have better knowledge of approaching a system that exhibits disparate features at different levels, we are able to derive an operational mechanism. Due to this reason plastic changes anticipated at the synapses become a weak candidate capable of explaining disparate findings from different levels. Reaching the correct solution implies that it can explain findings from all the levels of the systems to such an extent that we will be confident in replicating the mechanism in engineered systems, which should be the gold standard criterion in understanding the system.
We have to use all the findings from different levels of operation of the nervous system and work hard to find the solution that can remain invariable under all the conditions. It is hard; but this is what we have to do to get to the solution. In this approach, we should be ready for the following. 1) Whatever is the solution that can explain all the findings, we should be ready to tentatively accept it and try to verify it further. 2) Always consider the solution as a hypothesis until we use a large number of triangulations to confirm its accuracy. Once we get exhausted and fail to reject the hypothesis, we should be accepting it as further testable hypothesis. 3) Once we agree that there can be no other way that this system can function and is in agreement with all the expected features of an evolved system, then we should be ready to accept it. So, let us begin.
In order to become successful in solving a system, we have to include all the variables within different non-redundant linear equations (findings) of the system. This is a basic principle for success. Ignoring any single variable will not allow us to solve the system. The main function of the nervous system is generation of first-person inner sensation, within it (which we call as “mind”). Therefore, we have to include a variable for first-person inner sensations within the equations (findings) from appropriate levels for solving the system. Findings from the following levels are to be examined. 1) Systems, 2) Behavior, 3) First-person inner sensation, 4) Electrophysiological, 5) Cellular, and 6) Biochemical. By listing major findings from each level and the major constraint that they bring (Table 1), we will be able to derive a solution for the system.
From the above list, we can see a new level – first-person inner sensations - is introduced. This is of paramount importance in the case of the nervous system. Without this, we will not be able to find a mechanism that generates first-person inner sensations. This is the unique property of the nervous system that makes it different from all other systems in the body. So the real challenge is to understand at what locations and by what mechanism does the system generate first-person inner sensations. It will also help us to understand how this function is related to other features of the system – for example behavior. We currently use behavior to study several higher brain functions such as perception and memory and interpret the results. We have been consistently failing to solve the system since we haven’t taken into consideration the variable of first-person inner sensation while solving the system. So here we are using this level and trying to generate equations that contain this variable. What we meant by this in this approach is to define the relationship between findings from different levels with that of the generation of first-person inner sensations. For example, if a drug blocks memory retrieval as evidenced by lack of behavioral motor action indicative of memory retrieval, now we have to consider that the drug is blocking either generation of first-person inner sensation or its connected pathway towards the behavioral motor action.
By continuing the above approach, we hope to clarify the pathway of generation of inner sensations and its relationship with behavior. We also hope to understand how this pathway is generated during learning so that it can be reactivated at the time of memory retrieval. We should also make sure that the unique solution for the system should be compatible with all the previous experimental observations. All these functions are expected to operate at physiological time-scales of milliseconds. We must also maintain a low threshold for immediately verifying the validity of the arguments in any hypothesis of brain functions and if found tenable, it should be subjected to further verification.
Should we think about generation of unitary mechanisms and their integration to generate internal sensations?
In systems of the body that need generation of a very large number of outputs (products) using finite resources, these systems use the power of combinatorial effect by using unitary mechanisms. A typical example is the generation of nearly 1011 specific antibodies against the very large number of anticipated antigenic molecules from the environment by a combinatorial mechanism using finite number of variable (V), joining (J) and sometimes diversity (D) gene segments (Tonegawa, 1983; Janeway et al., 2001). This is possible by the common ability of different DNA segments to undergo recombination. Another example is the ability to make very large number of protein molecules using 20 different amino acids. This is achieved by the common properties of amino acids to form peptide bonds on their two ends. Similarly, these amino acids are formed from 4 different nucleotides having the common property to form phospho-diester bonds on their two ends. In summary, whenever a system has to generate very large number of outputs using finite resources, it is most likely that the system has selected a mechanism that utilizes a combinatorial mechanism. Since an infinite number of memories are expected to get generated using a finite number of neuronal processes, it is reasonable to assume that memory is formed from unitary mechanisms and their natural integration is occurring at physiological time-scales. In this context, it is most likely that the system is utilizing unitary mechanisms with the common properties that will allow them to get bonded together. In the case of memory, it is reasonable to expect generation of units of internal sensations that get integrated to generate internal sensation of memory in response to specific cue stimuli. When search is conducted based on the assumption that unitary mechanism operates, it is necessary to find a learning mechanism from which units of internal sensation can be induced and a mechanism that integrates these units to generate internal sensation of memory.
Explain derivation of semblance hypothesis in simple words?
Since the nature of retrieved memory changes as we keep changing the cue stimulus slightly, it indicates that the changes in cue stimulus are capable of inducing specific units of internal sensation. The induction of internal sensation occurring in physiological time scales requires explanation for a feasible cellular mechanism. Since the induced first-person internal sensation is virtual in nature, the aim of the hypothesis building was to examine the system for specific properties and mechanism that can induce such a function. The mechanism should operate by a simple mechanism and should be operating universally to explain similar functions in members of different species of animals. When the hypothesis was developed, care was given to make sure that it fits very well with the constraints offered by a large number of findings given in Table 2 on the front page.
The derivation of the hypothesis has two major stages. Each stage consists of few steps that are numbered.
Stage I
The derivation of the hypothesis has two major stages. Each stage consists of few steps that are numbered. This stage seeks explanations for the changes occurring during associative learning that can be used at a later stage to retrieve first-person internal sensation of memory. This can be approached by different methods. Here, two methods of approach are given. In both methods, few requirements need to be met, which are based on the following assumptions. a) Generation of an infinite number of internal sensations using a finite number of neuronal processes necessitates a combinatorial process occurring from unitary mechanisms, and b) The system should allow binding of the units of internal sensations. For this to occur, there should be a mechanism that holds the structure-function units together (binding property).
Method 1:
1. For the purpose of the derivation of the hypothesis, memory is viewed as a virtual inner sensation of a sensory stimulus since the sensory inputs from the item memorized is not present during the retrieval of memory.
2. We store thousands of memories. A specific internal or external cue stimulus is required to retrieve a specific memory.
3. Let us now conduct an imaginary experiment. Let us look at a yellow-colored pen. While looking at it, let us assume that a specific set of 105 synapses (out of the total 1015 synapses in our brain) was activated at different orders of neurons (1st order being the order close to the sensory level). If we can specifically stimulate the set of those specific 105 synapses, we can reasonably assume that we are likely to memorize/ visualize that yellow-colored pen.
4. How can we activate a specific set of 105 synapses out of the total 1015 synapses? Saying in a different way, how can we selectively activate each of those 105 specific synapses from a set of 1015 synapses for retrieving the memory immediately after associative learning? If we know how we can activate one of those 105 specific synapses that identify the item to be memorized, then we can extend the same mechanism to all the 105 synapses.
5. Alternatively, we can address the issue in a modified way. What is the minimum requirement that satisfies activation of a synapse? Activation of the postsynaptic terminal (dendritic spine or spine) can be taken as the equivalent of activating a synapse since the activation of a postsynaptic terminal takes place after the arrival of an action potential at its presynaptic terminal.
6. Since there is no sensory stimulus available from the item to be memorized, we cannot anticipate any action potential reaching at the presynaptic terminals of the routes through which it is supposed to propagate. Therefore, we need to activate the postsynaptic terminals of the synapses through which stimulus from the item (whose memory is to get retrieved) had passed before. This should occur in the absence of arrival of any action potentials at those presynaptic terminals during memory retrieval.
7. Activation of a postsynaptic terminal without the arrival of an action potential at its presynaptic terminal (at the synaptic level) can represent the idea of evoking a virtual sensation of a sensory stimulus (at the systems/behavioral level). In other words, the cue stimulus is expected to activate a specific set of postsynaptic terminals that can evoke the virtual sensation of a sensory stimulus arriving from the learned item. After the learning, can the cue stimulus activate the postsynaptic terminals of the synapses of the path through which the learned item had propagated before?
8. The above arguments get further support from the fact that lateral entry of activity of certain areas of the brain either artificially or by pathological conditions can induce virtual internal sensations in the form of hallucinations with a compelling sense of reality (Selimbeyoglu and Parvizi, 2010).
9. At this point, we come across with two key questions. 1) Can we activate the postsynaptic terminal of a synapse in the absence of the arrival of an action potential at the presynaptic terminal? 2) How can we choose to activate those 105 specific postsynaptic terminals from the total 1015 synapses for specific activation immediately after associative learning? What we have is a specific cue stimulus that activates a specific set of synapses. We can now arrive at a simple question at the synaptic level: “How can we activate a specific set of 105 postsynaptic terminals that would otherwise be activated by the item whose memory is to be retrieved in the presence of the activation of the specific set of synapses by the cue stimulus?
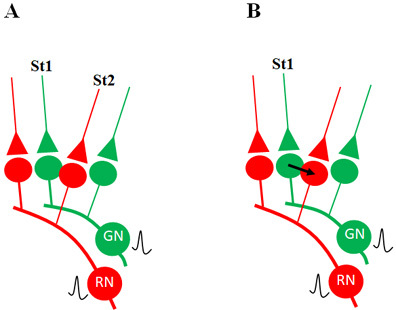
10. Let us assume that the cue stimulus evokes activation (depolarization) of the postsynaptic terminals through which activity from the learned item pass through. Then, it is reasonable to argue that some of the synapses through which activity spreads from the cue stimulus should be physically close enough to some of the postsynaptic terminals through which activity from the learned item passed through at the time of learning. At the time of memory retrieval, a mechanism should exist that can cause the spread of activity from the synapses of the cue stimulus to the postsynaptic terminals of the item whose memories are retrieved (Fig.1).
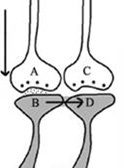
Figure 1. Illustration of the hypothesized depolarization spread during retrieval of memory. During retrieval, the cue stimulus reaching presynaptic terminal A depolarizes its postsynaptic membrane B, and the depolarization spreads to postsynaptic membrane D. This can only happen, provided there is a functional LINK between the postsynaptic terminals B and D. Therefore, we can assume that a functional LINK is required to be formed between postsynaptic terminals B and D during learning.
Method 2:
1. Let us imagine that two sensory stimuli, namely stimulus 1 and stimulus 2 undergo associative learning. At a later time when stimulus 1 (cue stimulus) arrives, it is expected to induce the internal sensation of memory of the second stimulus 2. For this to happen, it is necessary that some changes should occur at the locations of convergence of stimulus 1 and stimulus 2 at the time of learning. (Note that hippocampus known as an area of the brain associated with learning and memory receives inputs from all the different sensory modalities after 3 to 5 orders of neurons from the sensory receptor level). Now, let us examine what changes should be occurring at the location of convergence between two sensory stimuli at the time of learning. What should be the critical change occurring during learning between the synapses activated by stimulus 1 and stimulus 2? Between what locations of the synapses that these changes should take place? The interaction should take place between those sub-synaptic locations that will enable retrieval of memory of the second stimulus when the first stimulus arrives and vice versa. In this regard, interaction taking place between the postsynaptic terminals of stimulus 1 and stimulus 2 is suitable. (This was arrived by examining different sub-synaptic areas to find properties that endow them to generate units of internal sensation by trial-and-error method. This is described in section II). The interaction between the postsynaptic terminals was named as inter-postsynaptic functional LINK (Fig.2). The term "functional" is used to indicate that the formation of the LINK is a function of the activities arriving at the postsynaptic terminals activated by stimulus 1 and stimulus 2 during associative learning. At the time of memory retrieval, reactivation of the inter-postsynaptic functional LINK is a function of arrival of activity, from either stimulus, at one of their corresponding postsynaptic terminals. The term LINK is written in capital letters to indicate that it is the key element of the hypothesis.
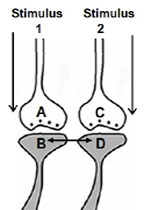
Figure 2. Ihe illustration shows the formation of hypothesized functional LINK between the two postsynaptic membranes B and D during associative learning between stimulus 1 and stimulus 2.
Different types of inter-postsynaptic functional LINKs formed during associative learning
The inter-postsynaptic functional LINKs formed during associative learning can be of different types:
a. Those that are formed by removal of water of hydration between the postsynaptic terminals, which will allow abutting of the membranes. This requires very high energy and will lead to a rapid reversal of the functional LINK. This can provide sufficient learning-induced changes that can last only for a short period of time responsible for working memory.
b. Strong interaction between the postsynaptic terminals can lead to reversible partial hemifusion between the postsynaptic terminals. This can explain the retention of learning-induced mechanism for more time.
c. Further interaction can lead to reversible complete hemifusion between the postsynaptic terminals that will enable its retention for much more time.
d. If the complete hemifusion can be retained for some time, it is likely that the stabilizing mechanisms can result in long-term maintenance of this.
Inter-LINKing spines are expected to belong to different neurons
At this juncture, it is paramount to understand the origin of the spines that are getting inter-LINKed. According to the studies based on synaptic plasticity thesis, either the spines of a single neuron cluster together at dendritic branches or the synapses at the spines to a single neuron make interactions (Govindarajan et al., 2006; Stuart and Spruston, 2015; Bloss et al., 2018), which are thought to be responsible for the integration of the inputs onto a single neuron.
In contrast to the above hypotheses, the present work approached the problem differently. The inter-LINK formed during associative learning is expected to generate first-person internal sensation at physiological timescales at the time of memory retrieval. Along with this, it is also expected to generate motor activity corresponding to the retrieved memory. This leads to the question, “To which neuron/neurons should the inter-LINKing spines belong, so that they can maintain specific outputs associated with each of the associatively learned sensory inputs?” The immediate answer is that they should belong to different neurons (Fig.3). Moreover, since the mean inter-spine distance is even larger than the mean spine diameter (Konur et al., 2003), the inter-LINKing postsynaptic terminals should belong to different neurons. This is expected to be the general rule. There could be exceptions; for example, when axonal terminals of newly formed granule neurons form synapses with a fixed number of dendritic spines of a CA3 neuron (Fig.4).

Figure 3. A) Sensory stimulus 1 and 2 activate sensory receptors and these activities propagate through several orders of neurons. For associative learning between these two sensory stimuli to occur, it is expected that they converge somewhere along their paths. Let us imagine that each stimulus has their specific motor output (shown that last order of neurons have their outputs to muscle fibers). The neuronal processes at the location of convergence are expected to generate a functional LINK between the processes of these two paths. Following associative learning, each stimulus is expected to elicit motor response reminiscent of the second stimulus along with generation of internal sensation of the second stimulus. Now the question is "Where can the inter-postsynaptic functional LINK (IPL) occur between the two paths?" B) Can the sensory inputs reach two closely located spines of a neuron? Is there any issues with such an arrangement? Even though an IPL can be formed between these spines theoretically, there are some practical difficulties. a) The outputs of the associatively learned sensory stimuli 1 and 2 have to go through the same neuron N. Therefore, this arrangement cannot manifest motor actions expected of a conditioning paradigm. b) The observation that the average inter-spine distance is more than the average spine diameter (Kamme, 2003) informs that inter-spine interaction has to take place through the dendritic branch. Since there are no known electrically isolated cables between the spines through the dendritic branch, this setup does not have the capability to provide a universal operational mechanism. C) This poses the question, "When one sensory input arrives at one spine of a neuron, what should be the route through which the second sensory stimulus arrive to accomplish the features expected of a) a learning mechanism from which internal sensation of memory can be induced, and b) output activity that directs motor action reminiscent of the arrival of the second stimulus?" D) The next feasible configuration of arrangement is to have an interaction between the spines neurons that belong to separate neurons. The figure shows convergence of stimulus 1 and stimulus 2 onto the spines of two separate neurons (marked N in different colors) providing separate motor outputs. If those spines are abutted to each other and if their interaction during learning can provide an inter-spine mechanism that can be reactivated by one of the stimuli (after learning) to induce the internal sensation of memory of the second stimulus and if the inter-spine mechanism has different half-lives to explain short- and long-term memories, then it is a suitable candidate mechanism. In addition, it is expected to find a strong connection between the inter-spine interaction and the narrow range of frequency of oscillating extracellular potentials. S: sensory stimulus; N: neuron. Inter-postsynaptic functional LINKs can be viewed as biological equivalents of K-lines proposed by Minsky (Minsky, 1980).
.
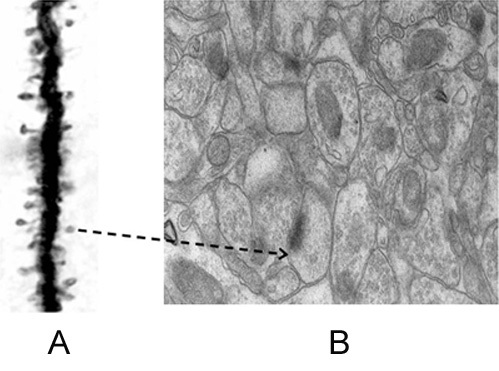
Figure 4. Figures showing the importance of making an inference that it is most probable that the nearest spine to a spine on the dendritic branch of a neuron is a spine that belongs to another dendrite (likely belonging to another neuron). A) Golgi staining showing a dendritic branch that has spines on them. These are the inputs to a neuron. The output terminals of the preceding neurons that synapse with these spines will not take any Golgi stain. We must assume that they are present adjacent to them (but both can be seen in electron microscopic picture shown in Figure B). Nearly any 140 such inputs arriving at the neuronal axon hillock fire that neuron resulting in propagation of a signal to all its output terminals. B) An electron microscopic picture showing how crowded are the neuronal processes (and other cells). The extracellular matrix space between neuronal processes (and glial cells) that we assume to act as an insulating medium preventing spread of signals between different neurons that have no connections is very thin. Arrow: Arrow from a spine on figure A is shown towards a spine in figure B. Note that it has postsynaptic density (PSD) (a dense dark area) & cellular process adjacent to it is a presynaptic terminal with synaptic vesicles inside (Please see Fig.9 for more clarity). Since mean inter-spine distance is more than mean spine diameter (as seen from figure A), nearest spine to a spine on a dendritic branch is a spine that belongs to another dendrite. If we expect a brain function through spine-spine interaction and if it needs output from a different neuron (as demanded by classical conditioning experimental results), then the nearest spine to a spine on a dendritic branch is a spine that most probably belonging to a dendrite of another neuron. No scale bars used.
Inter-postsynaptic functional LINKs can be viewed as biological equivalents of K-lines
K-lines were proposed as the key operational change occurring at the time of associative learning (Minsky, 1980) that is expected to provide the necessary function during memory retrieval. This proposal came as a result of attempts to understand natural intelligence that can be translated into engineered systems.
Stage II
In the next stage, the basic units of semblances occurring at the functionally inter-LINKed postsynaptic terminal are derived. Let us examine the effect of the arrival of the stimulus during memory retrieval. Let stimulus 1 arrive as a cue stimulus (Fig.5). It arrives at the synapse A-B. Postsynaptic potential at B propagates through the inter-postsynaptic functional LINKs and reach towards postsynaptic terminal D. As discussed in Method 1, the arrival of the stimulus 1 (cue stimulus) happens only infrequently. Therefore, when second postsynaptic terminal D is depolarized incidentally in the absence of the arrival of an action potential at its corresponding presynaptic terminal C, then postsynaptic terminal D is expected to get the cellular hallucination that it is receiving sensory inputs through its presynaptic terminal C, resulting in “semblance". This can induce units of virtual inner sensation of memories at the time of memory retrieval and can meet the expectations of a mechanism for memory (Minsky, 1980), if there is a specific operational logic at this location. Before examining the operational logic for the generation of internal sensations, we need to answer two questions. 1) How can a cellular hallucination (semblance) get induced at inter-LINKed postsynaptic terminal D that was previously activated by the item whose memory needs to get retrieved? 2) What is the sensory content of this hallucination?
.
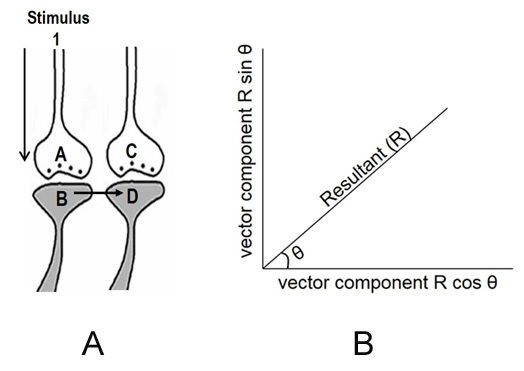
Figure 5. Extracellular ionic changes during synaptic transmission and propagation of depolarization along the IPL contribute vector components to oscillating extracellular potentials. A) During retrieval, the cue stimulus reaching presynaptic terminal A depolarizes its postsynaptic membrane B, re-activates the inter-postsynaptic functional LINK. In this manner, depolarization spreads to postsynaptic membrane D evoking cellular hallucination at the postsynaptic terminal D of the arrival of sensory stimuli at its presynaptic terminal C. This is named semblance. B) The propagation of potentials at the synapse A-B and through the inter-postsynaptic LINK B-D provides ionic changes in the extracellular matrix space that contribute vector components for the oscillating extracellular potentials.
The propagation of potentials through the synapse A-B and the IPL B-D provide vector components that are responsible for contributing to the oscillating extracellular potentials (Fig.5B).
When the related learning events continue, one of the postsynaptic terminals that already took part in a previous learning event (either B or D in the Fig.5) will be used to form functional LINKs with the postsynaptic terminals of the neighboring synapses (seen as additional postsynaptic terminals on the right side of the postsynaptic terminal D in the left panel, Fig.6). As this process continues, it will result in the formation of islets of LINKed (LINKable/ re-activatible during retrieval) postsynaptic terminals (right panel, Fig.6).
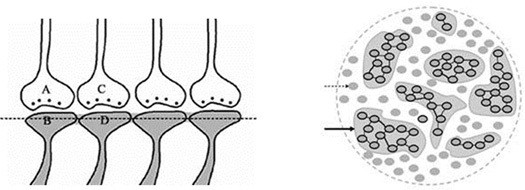
Figure 6. Left panel: An illustration showing the formation of islets of LINKed postsynaptic terminals. Continued learning events following the initial learning event can lead to the formation of multiple inter-postsynaptic LINKs between the involved postsynaptic terminals (dendritic spine heads). Only two presynaptic terminals (A and C) and two postsynaptic terminals (B and D) are marked. Assume that there are several postsynaptic terminals arranged in a horizontal plane. The dotted line shows a cross-section across the inter-LINKed postsynaptic terminals. Right panel: A hypothetical cross-sectional view of LINKed postsynaptic terminals of the synapses in one horizontal plane in a brain region (see the horizontal dotted line across the postsynaptic membranes in the left panel); in this illustration, we imagine that all the postsynaptic membranes are in the same plane. Postsynaptic membranes are shown in small dark circles (broken arrow). When learning occurs, functional LINKs between activated postsynaptic terminals can be established. Continued learning using any of those synapses will increase the number of interconnected postsynaptic membranes forming islets of functionally LINKed postsynaptic terminals (solid arrow). Multiple LINKs between the postsynaptic terminals in an islet can cause spread of postsynaptic potentials across the islet. The individual islets are expected to be functionally separate from each other.
It is expected that basic units of semblances take place at the functionally inter-LINKed postsynaptic terminal (see Fig.5). Here, we need to answer two questions. 1) How can a cellular hallucination (semblance) get induced at the inter-LINKed postsynaptic terminal D that was previously activated by the item whose memory needs to get retrieved? 2) What is the sensory content of this hallucination?
What is the logic behind the generation of cellular hallucination (semblance)?
Semblance is the mechanism by which virtual internal sensations are being created. Searching for a cellular location where such a mechanism can be formed resulted in arriving at the requirement for inter-postsynaptic functional LINK. In figure 5, when cue stimulus arrives at the postsynaptic terminal B and re-activates the inter-postsynaptic functional LINK, it activates the postsynaptic terminal D. What makes the postsynaptic terminal to have a cellular hallucination (semblance) that it is receiving activity from its own presynaptic terminal C? The logic can be explained as follows. By default, the postsynaptic terminal D is normally activated by its presynaptic terminal C. To make sure that this is the case, it appears that the Mother Nature has designed an excellent method. There is a continuous quantal release of neurotransmitter molecules from the synaptic vesicles of the presynaptic terminal C even during periods of rest (and sleep). These provide regular arrival of miniature potentials at the postsynaptic terminals. The combined effect of all these potentials is represented by the miniature excitatory postsynaptic potentials (mEPSPs or “minis”). The fact that it is not possible to completely block mEPSPs “even in experimental conditions” indicates that it is a highly conserved default operation of the nervous system. Another necessary condition is the maintenance of oscillatory neuronal activity. The finding that electrical stimulation of the visual cortex produces a visual percept (phosphene) only when high-frequency gamma oscillations are induced in the temporo-parietal junction (Beauchamp et al., 2012) emphasizes the role of oscillating neuronal activity as a system requirement for the semblance formation for creating internal sensations. The lateral spread of activity through the inter-postsynaptic functional LINKs can contribute towards the horizontal component of the oscillating potentials and the synaptic potentials between vertically oriented neurons in the cortex can provide the vertical component. Since inter-postsynaptic spread of potentials occurs perpendicular to the trans-synaptic spread of potentials, this general feature can explain the wave form of oscillating potentials in all other regions in the nervous system, especially where sensory inputs converge.
What is meant by tricking the inter-LINKed spine to hallucinate?
The inter-LINKed spine heads, like the heads of any other spines (postsynaptic terminals), are continuously being depolarized by quantally-released neurotransmitter molecules all the time, including during sleep. This sets the dominant state of the system that allows any laterally arriving depolarization through the IPL to trick the inter-LINKed spine to hallucinate that it is receiving sensory inputs from the environment through its presynaptic terminal. Are there any real-life examples for the occurrence of such a hallucination? This has been asked recently by few readers. Here are two examples and they highlight the importance of maintaining a dominant state to trick the system to hallucinate.
1. First example is the principle underneath the success of pick pocketers for successfully doing their job! When I was in grade six, we had to read from “The Adventures of Tom Sawyer” by Mark Twain. Young Tom Sawyer along with a group of boys got training to pickpocket. Here, Tom Sawyer had to dissuade the attention of the people by taking advantage of either introducing alternate sensory stimuli or wait for their natural occurrence so that he could take a wallet from someone’s pocket without their attention. Here is a modern version of pick pocketing (and how to avoid getting pick pocketed!). Watch the events of pick pocketing in this video to see how a regular background stimulus (stimuli arriving from regular movements while walking up or down the stairs) can trick someone to think that the stimuli during pick pocketing is perceived only as a normal stimulus (Video; Fig.7). In the video, we can see that pick pocketers are successful when the victim walks up or down the stairs.
.

Figure 7. A cartoon showing pick pocketing a walking person by another person. The movements at the gluteal region remain dominant so that any stimuli acting in the direction will be perceived only as part of the natural movement. (Figure taken from Wikipedia. No original source could be found).
It can be noticed that the body movements that happen as one climbs up or walks down the stairs act as the new normal background sensations during which pick pocketers can trick one’s sensory system to perceive the pick pocketing only as the movement of one’s body as one climbs up or walk down the stairs. The thief is taking advantage of the new dominant state of the system where continuous new sensory stimuli of moving through the stairs to trick that system to pickpocket!
2. A second example is that of giving injections to animals. One of the tricks here is to first pat the location where one wants to inject (Fig.8) and then tap at that location with much more intensity several times before one can put the needle at that location. If you tap 10 times, you can successfully put the needle on the 11th time without animal’s attention. You have to continue to tap again for another 10 times before you can put the needle for a second time at the same location without animal’s knowledge (Video - watch between 7 & 7.2 minutes). The bottom line is that to trick the system to hallucinate that it is only the tap and not the needle, you have to make the sensory stimuli arriving from "tapping" as the dominant normal state of the system. In this background state, you can successfully put the needle (trick the animal to sense that the needle is only another tap).
.

Figure 8. Tapping on the skin over the internal jugular vein of a horse as a preparation before an intravenous injection. We can see that the veterinary doctor is tapping over the vein at the injection site several times. Also the pressure over the vein by the left thumb provides some continuous sensory stimuli so that arrival of sensory stimuli from this region (dermatome) is the new norm for the nervous system. In this context, the needle goes in without causing pain (Figure taken from Wikipedia. Source could not be traced).
The above two examples are not perfect. But they can give some good idea how a system can be tricked to hallucinate, provided you can maintain a dominant state. Now the question is how can the nervous system end up operating in this manner? If we look carefully, synapses are having quantal release from the presynaptic vesicles all the time, which depolarize the spine heads including that of the inter-LINKed spines. There are no toxins on the Earth that can completely block this quantal release (& we hope that we will not bring any toxins from any other planets or satellites to the Earth!!). In a system where the synaptic junctions are having continuous quantal release, accidental coincidence during the early evolutionary stages might have brought two spines (postsynaptic terminals) to abut each other and form an IPL during simultaneous arrival of two stimuli from an item. Later, arrival of one of the stimuli allowed propagation of the postsynaptic potentials to depolarize the inter-LINKed spine from a lateral direction, tricking this inter-LINKed spine to hallucinate that it is receiving sensory inputs from the environment through its presynaptic terminal. This possibly started providing survival advantage to the animal in instances when the fastest (light) or first (smell or sound from a curved location where light cannot curve) arriving stimulus reach the nervous system. This property continued to get modified over generations to form the operational mechanism of the nervous systems. Article
Also note the importance of maintaining a dominant state of the system for tricking the system to hallucinate about the sensory content of the associatively learned second item. In the above described case of injecting a horse, if you tap 10 times, you can successfully put the needle on the 11th time without animal’s attention. You have to continue to tap at again for another 10 times before you can put the needle for a second time at the same location without animal’s knowledge. This means that you have to reset the background state to a state where you can trick the system to hallucinate that it is only the regular tap before giving the second injection. Even though, this is not a perfect example, it embraces the idea to certain extent. This has similarities to sleep in animals. During the evolution of the nervous system on Earth, where there is day and night, sleep provided much of the needed opportunity to put the system back to having a dominant state where depolarization of the spines heads by the continuous quantally-released neurotransmitter molecules became the dominant state of the system. This explains the substantive (not just indispensable) nature of sleep in maintaining the system. Article
What is the sensory content of the cellular hallucination (semblance)?
Cue stimulus activates postsynaptic terminal B that leads to re-activation of inter-postsynaptic functional LINK and activates the postsynaptic terminal D that was previously activated by the item whose memory is getting retrieved now (Fig.9). At postsynaptic terminal D, this leads to a semblance of activity arriving from the sensory receptors through neuron Z. Neuron Z is normally depolarized by activating a set of axonal terminals of the neurons in order 4 that synapse to neuron Z’s dendritic spines (postsynaptic terminals). The spatial summation of nearly 40 or the temporal summation of less than 40 EPSPs (from nearly 40 postsynaptic terminals (dendritic spines) out of the nearly 4×104 postsynaptic terminals of each neuron) triggers an action potential at neuron Z’s axon hillock (Note that the number of postsynaptic terminals (dendritic spines) for a neuron varies. In the hippocampus, we expect that the excitatory neurons have postsynaptic terminals in the order of 104). In the same way, the neurons in set {Y} in turn receive synaptic transmissions and spread of activity through functional LINKs from a set of neurons {X} in neuronal order 3. By continuing the extrapolation in a retrograde fashion towards the sensory level, it will be possible to determine the set of sensory receptors {SR} whose activation could theoretically cause the activation of postsynaptic terminal D.
.
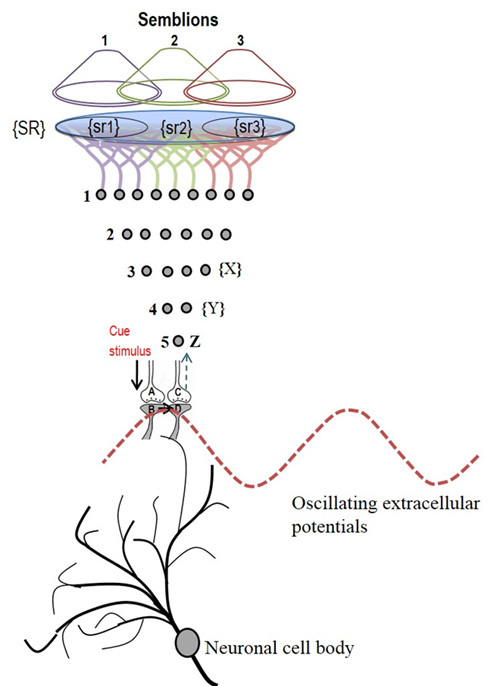
Figure 9. Schematic representation of sensory elements induced during the activation of a synapse. The gray circles represent neurons. The numbers on the left side of the neuronal orders denote their position in relation to the sensory receptors. Neuron Z is shown in neuronal order 5. During memory retrieval, a cue-stimulus reaching presynaptic terminal A depolarizes its postsynaptic membrane B and the resulting EPSP at postsynaptic terminal B re-activates the functional LINK that activates postsynaptic membrane D. When postsynaptic membrane D is depolarized, it evokes the cellular hallucination of an action potential reaching its presynaptic terminal C. This is called synaptic semblance. Note that presynaptic terminal C belongs to the neuron Z. Either synaptic semblance occurring at postsynaptic terminal D or random activation of neuron Z produces the hallucination that it is receiving input from the set of neurons {Y} that synapse to it. The set of neurons {Y} are activated by the activation of the set of neurons {X}. The set of neurons {X} in turn are activated by the set of neurons in the neuronal order above it. (Recurrent collaterals and projection neurons can also activate a higher order neuron. For simplicity, these are not shown). Continuing this extrapolation towards the sensory level identifies a set of sensory receptors {SR}. Stimulation of subsets of sensory receptor sets {sr1}, {sr2}, and {sr3} from the set {SR} may be capable of independently activating neuron Z. The dimensions of hypothetical packets of sensory stimuli capable of activating the sensory receptor sets {sr1}, {sr2}, and {sr3} are called semblions 1, 2 and 3 respectively. These semblions are viewed as the basic building blocks of the virtual internal sensations of memory. A cue stimulus can cause postsynaptic terminal D to hallucinate about any of the semblances 1, 2, 3 or an integral of them. Activation of postsynaptic terminal D by the cue stimulus can lead to the virtual internal sensation of different combinations of semblions 1, 2, 3 or an integral of them. The method of integrating the semblions that match can with the internal sensations induced by the cue stimulus with that of the item whose memory is retrieved can be determined by computational studies. Note that the potentials through the synapse and perpendicularly located IPL contribute vector components to the oscillating extracellular potentials (marked by the waveform) (Modified from Vadakkan, 2011).
Dimensions of internal sensations resulting from the lateral activation of postsynaptic terminal D can be understood from the nature of the sensory stimulus that can activate sensory receptors in the set {SR}. It is likely that activation of subsets of a minimum number of sensory receptors from {SR} (example, {sr1}, {sr2}, and {sr3} (Figs.9&10) is sufficient to activate postsynaptic terminal D. Therefore, a hypothetical packet of minimum sensory stimuli called “semblion” capable of activating one of the above subsets of sensory receptors that can activate postsynaptic terminal D is hypothesized as the basic unit of internal sensation of memory.
.
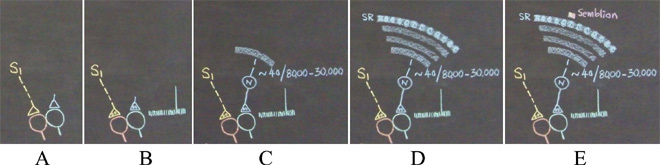
Figure 10. An alternate description is shown in the figure below. A) What can spark a unit of internal sensation when stimulus1 (S1) arrives at one spine of the inter-postsynaptic functional LINK that was formed with the spine of another neuron at the time of learning? The background conditions at the inter-LINKed second spine is that a) the spine head is getting continuously depolarized by the quantal release of neurotransmitter molecules from its presynaptic terminal all the time, which is shown by small vertical lines in the figure, and b) large postsynaptic potential generated by the intermittent arrival of a volley of neurotransmitter molecules when an action potential arrives at its presynaptic terminal (shown by a large vertical line). B) The activation of the inter-LINKed spine from a lateral direction sparks a hallucination that it is receiving a sensory input from the environment through its presynaptic terminal. By making a retrograde extrapolation from the inter-LINKed second spine's presynaptic terminal, we can identify the sensory receptors from where the activity can arrive. Everything here on wards is not associated with neurotransmission. It is virtual in nature. Even though any set of 40 inputs arriving from locations close to the soma (or nearly 140 random inputs arriving from anywhere from the dendritic tree) out of tens of thousands of its inputs (marked in the figure as 8000 - 30,000) can fire the neuron N, the retrograde extrapolation should include all the inputs of neuron N. C) Continuing this process to the level of the sensory receptors identifies a large set of sensory receptors {SR}. From this, a large number of sets of minimum sensory stimuli whose activation can activate subsets of the large sensory receptor set {SR} can be found. D) This extrapolation is continued towards the lower orders of neurons until it reaches the level of the sensory receptors. This will identify all the sensory receptors. The content of the hallucination occurring at the inter-LINKed second spine is about the sensory stimuli stimulating these sensory receptors. Here, we have to think again and ask, "Is it necessary to stimulate all these receptors for an action potential to arrive at the presynaptic terminal of the inter-LINKed spine in real life?" This need not be necessary. In fact, activation of a small subset of these receptors will be able to generate an action potential of the presynaptic terminal's neuron. In other words, content of hallucination at the inter-LINKed spine can be of a sensory stimulus that can activate a fraction of sensory receptors {SR} that are drawn as round dense areas on the sensory receptor (SR) layer in the figures D and E. The content of hallucination can be a hypothetical packet of minimum sensory stimuli activating a minimum set of sensory receptors. E) At the top of this picture a set of minimum sensory stimuli that forms the content of the hallucination (internal sensation in the absence of arrival of a sensory stimulus), which is called a "semblion" is shown above one of the sensory receptor subset.
As the cue stimulus passes through different functional LINKs, it evokes a large number of semblances as explained above. Once these possible semblions are identified, their integration can be carried out to obtain a net semblance that matches the sensory characteristics of the item whose memory is retrieved. Attempts to match the different integration products from the semblions with that of the sensory stimuli from the item whose memories are retrieved will lead to the discovery of the algorithm for neural computations for memory retrieval. The net semblance can exceed more than the threshold without any effect on the retrieved memory. As the functional LINKs get re-activated during memory retrieval, the expected spread of excitatory postsynaptic potential (EPSP) that occurs through some of these functional LINKs can be crucial in adding to the existing sub-threshold EPSP at the axonal hillocks of some neurons that are routinely activated by the oscillatory neuronal activities in the hippocampus and cortex as well as from baseline sensory activities arriving at many neurons. Since the number of functional LINKs continues to change (due to continued associative learning) over the lifespan of the nervous system, the characteristic features of the semblions are also expected to change gradually. This will lead to gradual changes in the net semblances for memory. Related learning can increase the number of LINKed postsynaptic terminals and increase semblance for memory. Absence of retrieval of a specific memory, lack of repetition of learning or lack of related learning will reduce the number of re-activatible inter-postsynaptic functional LINKs and will reduce semblance for retrieval of a specific memory. Along with the induction of semblances, the reactivation of inter-postsynaptic LINKs can also provide additional potentials to the inter-LINKed postsynaptic terminal that can lead to firing of the latter’s neuron if it is kept at a subthreshold activated level (Fig.11).
.
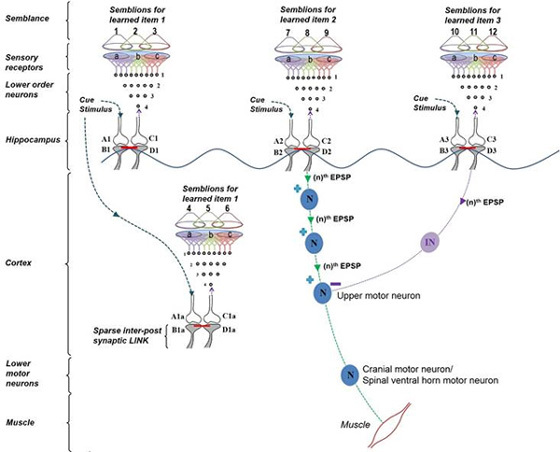
Figure 11. Diagram showing the formation of internal sensations and fine control of the motor activation by a cue stimulus. Oscillating neuronal activity results in the activation of many downstream neurons. They can be kept tonically inhibited under resting conditions (not shown) to subthreshold levels, such that they can be disinhibited at the arrival of one or a few excitatory postsynaptic potentials (EPSPs). There were two associative learning events that occurred previously with the cue stimuli. The first one was with items 1 and 2. After this first step of associative learning, the cue stimulus was retrieving memories of items 1 and 2. Note the reactivation of a sparse inter-postsynaptic functional LINK in the cortex. Along with retrieving memory of the second item, the cue stimulus also evokes a motor response using the motor neuron. At a later time, the same cue stimulus had undergone a second associative learning event with item 3. Following this second learning event, the cue stimulus evoked internal sensations (semblances) of learned items 1, 2 and 3. However, as the semblance for item 3 was evoked, it also resulted in an inhibition of the motor activity (note the output from postsynaptic terminal D3 provides inhibitory potentials to the upper motor neuron). This type of an event is an example of the behavioral inhibition occurring at the frontal cortices. The complexities of the internal sensations can be based on the nature of the cue stimulus, previous associative learning, and the type of the nervous system. Reward-induced associative learning may be facilitated by dopamine-induced enlargement of dendritic spines (Yagishita et al., 2014) that promotes possible inter-postsynaptic membrane hemifusion and its stabilization for a long period of time. Also note that the cue stimulus reactivates inter-postsynaptic functional LINKs at other cortical areas to evoke memories for learned item 1. Since the inter-postsynaptic functional LINKs are transient and need reinforcement for long-term persistence, the induction of a minimum number of inter-postsynaptic functional LINKs alone may not maintain the effect of learning for a long period of time. In the hippocampus, the reactivation of inter-postsynaptic functional LINKs in response to spatial stimuli is expected to induce semblances for memories associated with that space and the EPSPs arriving through the inter-postsynaptic LINK induce firing of subthreshold-activated CA1 neurons (place cells). This explains how spatial memories are associated with place cell firing. Formation of the circuits in this manner can explain the induction of internal sensations along with the simultaneous behavioral motor action. Note the formation of a sparse inter-postsynaptic functional LINK at the cortex, which can contribute to the specificity of retrieved memory (for a more complex path of its formation, see figure 9 in Vadakkan, 2015b). EPSP: excitatory postsynaptic potential. nth EPSP: the last EPSP necessary to achieve threshold EPSP to generate an action potential. Each motor action will evoke certain sensory stimulus in the form of proprioception that will act as a feedback stimulus to the system confirming that the motor action was executed. N: Excitatory neuron; IN: Inhibitory neuron. A and C: Presynaptic terminals; B and D: Postsynaptic terminals. Red line between B and D: Inter-postsynaptic LINK. (+) stimulation; (-) inhibition (Modified from Vadakkan, 2015b).
What is the nature of inter-postsynaptic functional LINK?
Different mechanisms for the formation of inter-postsynaptic LINKs are possible and are required to explain the formation of internal sensations of other higher brain functions that operate at different time-scales. These different types of inter-postsynaptic LINKs with varying half-lives are suitable to explain perception, working, short- and long-term memories. A description of some of them is given in Fig.12.
.
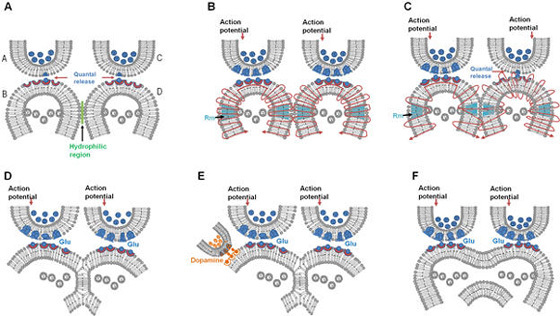
Figure 12. Different types of reversible inter-postsynaptic functional LINKs. A) Two abutted synapses A–B and C–D. Presynaptic terminals A and C are shown with synaptic vesicles (in blue color). Postsynaptic terminals (dendritic spines or spines) B and D have membrane-bound vesicles marked V containing subunits of AMPA receptor inside them. Action potential arrives at presynaptic terminal A releasing a volley of neurotransmitters from many synaptic vesicles inducing an excitatory postsynaptic potential (EPSP) at postsynaptic terminal B. From the presynaptic terminal C, one vesicle is shown to release its contents into the synaptic cleft. This quantal release is a continuous process (even during rest) that leads to the generation of very small potentials on postsynaptic membrane D. Note the presence of a hydrophilic region separating postsynaptic terminals B and D. When an action potential arrives at presynaptic terminal A, it activates synapse A–B and generates an EPSP at postsynaptic terminal B. The hydrophilic region prevents any type of interaction between postsynaptic terminals B and D. Very high energy is required for excluding the inter-postsynaptic hydrophilic region (Martens and McMahon 2008). B) Membrane expansion occurring at physiological time-scales can provide sufficient energy to exclude the inter-postsynaptic hydrophilic region, allowing close contact between the postsynaptic membranes in this region. This forms a transient inter-postsynaptic LINK that lasts only for a short period of time. During this short period of time, a cue stimulus-generated action potential arriving at synapse A–B reactivates this inter-postsynaptic functional LINK and spreads to postsynaptic terminal D and induces units of internal sensation at the inter-LINKed postsynaptic terminal D. This can explain working memory. C) Diagram showing formation of a partial inter-postsynaptic membrane hemifusion. These vesicles contain glutamate receptor subtype 1 (GluA1). Activity arriving at the synapse can lead to exocytosis of vesicles containing AMPA GluA1 receptor-subunits abutted to the cell membranes and expansion of the postsynaptic membrane at physiological time-scales. During exocytosis, the vesicle membrane gets incorporated into the postsynaptic membrane at locations of exocytosis making this region of the membrane highly re-organizable. This matches with the location where AMPA receptor subunits were shown to concentrate at the extra-synaptic locations extending up to 25nm beyond the synaptic specialization (Jacob and Weinberg 2014). Note the interaction between the outer layers of membranes of the postsynaptic terminals. Depending on the lipid membrane composition, the process of close contact between the membranes described in the above section B) can get converted to a partial hemifusion state. D) Stage of partial hemifusion can progress to complete hemifusion. The reversible partial and complete hemifusions are short-lived and can explain the necessary learning-induced changes responsible for short-term memory. Some of the hemifusion changes can get stabilized for different lengths of time. For example, insertion of a trans-membrane protein across the hemifused segment can maintain the inter-postsynaptic LINK until this protein gets removed. These changes can be responsible for long-term memory. E) Dopamine is known to facilitate motivation-promoted learning. In this diagram dopaminergic input to spine (postsynaptic terminal) B that results in latter's expansion, which will augment inter-postsynaptic LINK formation. This can explain the action of dopamine on learning. Furthermore, it can sustain the hemifused LINK for a long period of time, which may facilitate its stabilization. F) Hemifusion can advance to a complete fusion state in pathological conditions and it depends on several factors. Fusion of the postsynaptic terminals between two different neurons can lead to cytoplasmic content mixing and cytotoxic cell response. These include dendritic spine loss and eventually triggering of apoptosis leading to neurodegenerative changes. Note that excessive dopamine can lead to excessive expansion of the postsynaptic membrane and can lead to membrane fusion if other factors that resist this get compromised. Rm: membrane segment marked in Turkish blue shows area where membrane reorganization occurs (Figure modified from Vadakkan, 2015a, b).
Are there any experimental evidence supporting the presence of the inter-postsynaptic functional LINK?
New technologies are required to test for the presence of the close contact between the membranes by hydration exclusion (Fig.12B) in vivo. Another mechanism of inter-postsynaptic functional LINK is the reversible inter-postsynaptic membrane hemi-fusion. If this is correct, then examination of the membrane bilayers at locations where postsynaptic areas are close together is an opportunity to test the hypothesis. It is also true that at locations where (sensory) inputs converge, the extracellular matrix space is very minimal as observed by routing electron microscopic (EM) examination of these regions. At these locations, abutted postsynaptic membranes are expected to be seen. However, there are some hurdles. First, the membrane hemi-fusions are reversible. However, locations within the hippocampus that has already undergone many associative learning, stabilization of these hemi-fused areas (most probably by the insertion of trans-membrane proteins) are expected. Secondly, only a very small area of membrane hemi-fusion is required for the functional effect of the formation of inter-postsynaptic functional LINK. Since the area of the postsynaptic membrane surface that has to be examined for such small areas of membrane hemi-fusion is very large, dedicated EM studies by taking serial sections spanning an entire postsynaptic terminal is required.
Alternatively, examination of a large number of electron microscopic pictures of the hippocampal regions taken for other purposes can be tried. The limitation of this is the lack of resolution of the electron microscopic pictures to visualize the membrane double layer. In a recent EM work (Fig.13) with good resolution, it is possible to observe closely abutted areas, suggesting that they may lack inter-membrane extracellular matrix space. Since dehydration during the tissue processing contribute to these observations, inter-membrane close contacts with hydration exclusion need to be verified using new in vivo techniques. In the above figure, another finding is very striking. There are areas of two layers of hemi-fused membrane for short distances instead of four layers of the two abutting postsynaptic membranes. These are very unlikely to be caused by rotation of the membranes or changes during processing of the tissue. These short spans of reduced number of layers is what is expected by the hemi-fusion process and provide support for the hypothesis until further verifications are carried out. Multiple fused spine heads on a single spine neck seen on dendritic excrescences at the CA3 dendritic tree (Amaral and Dent, 1981; Chicurel and Harris, 1992; Frotscher et al., 1991) is a possible structural modification evolving from long-standing inter-postsynaptic functional LINKs.
.
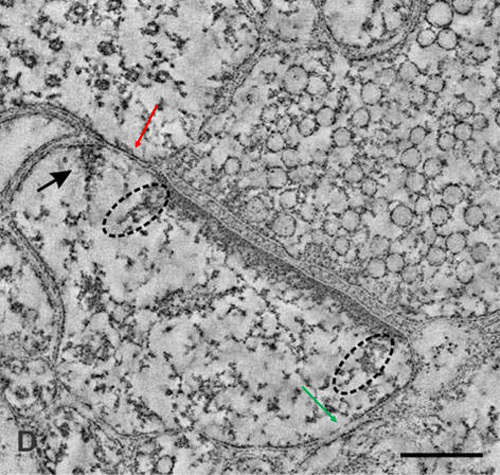
Figure 13. This is figure 4D from Burette A.C, Lesperance T, Crum J, Martone M, Volkmann N, Ellisman M.H, and Weinberg RJ (2012) Electron Tomographic Analysis of Synaptic Ultrastructure. Journal of Comparative Neurology 520 (12): 2697-2711. This figure is modified by inserting one red and another green arrow. The red arrow points towards a likely inter-postsynaptic area with only 2 layers of membrane instead of the expected 4 layers. This is a suspected area of inter-spine hemifusion. Note that that if the structure on which the red line is drawn is a spine, then that spine does not show postsynaptic density in the present section. If it is a spine, then it is reasonable to expect it to have postsynaptic density at the location where it synapses with another presynaptic terminal of possibly another neuron. This will then fulfill the expectations of an inter-neuronal inter-spine hemifusion. The green arrow points to a likely location where the close contact between the membranes is visible, which is likely a location of partial hemifusion. Even though tissue distortions during tissue processing and folded membrane are possibilities, such changes that can span for distances of only 100 nm is very unlikely. This observation needs further dedicated studies for its verification. Furthermore, since some of the cell processes are likely astrocytic pedocytes, dedicated studies are required to verify these observations. Scale bar = 100nm. In contrast to routine electron microscopic sections that use 5 micrometer sections, this study used sections of ~120 nm thickness. This tremendously increased the likelihood of finding suspected hemifused regions of length 100 nm. However, since it is necessary to observe sections along the linear axis of an expected linear structure, the finding of these structural patterns in a random EM section shows a high probability for its universal presence (due to the presence of multiple inter-spine interactions by one spine that leads to islets of inter-LINKed spines - see Fig.5 in this page) and warrants further verification.
Why didn't we discover these IPLs until now?
First, there was no reason to search for a mechanism of exclusion of water of hydration at the inter-neuronal inter-spine regions that are activated during associative learning. Secondly, no dedicated studies were carried out to image the lipid bilayers of an entire dendritic spine and its interactions with the abutting spines that belong to different neurons to examine the formation and reversal of inter-postsynaptic functional LINKs by a) exclusion of water of hydration between them, b) formation of inter-spine partial and complete hemifusion, and c) formation of inter-spine fusion in pathological conditions. Though sound simple, this is a huge undertaking with many challenges. Since the IPLs are expected span a length of only nearly 10nm length, ultra-structural details of entire spine membranes are necessary.
It seems that all the above steps used third-person observations. Where is the examination from a first-person frame of reference?
In Figure 9, the steps needed in finding out the sensory content of the cellular hallucination induced at the postsynaptic terminal D involves examination form a first-person frame of reference. It requires searching backwards from the postsynaptic terminal D towards the sensory receptor level to find out the subset of minimum sensory receptors whose stimulation can activate the postsynaptic terminal D. The minimum sensory stimuli required to activate this subset of sensory receptors constitute the semblion, which is the basic unit of internal sensation. The backward extrapolation from the postsynaptic terminal D towards the sensory receptor level to find out the packets of sensory stimuli is an implicit process taking place during the internal sensations of all the higher brain functions. In this examination, we observe the packets of sensory stimuli (content of the unit of internal sensation) from a first-person frame of reference.
How can we explain long term potentiation (LTP) in terms of the semblance hypothesis?
For a more detailed description, see published article
The semblance hypothesis was derived to explain plausible synaptic changes occurring during learning suitable for evoking virtual inner sensation of a sensory stimulus during memory retrieval. The operational principle of the formation of semblances resulting in memories is completely different from that of LTP; however, the formation of inter-postsynaptic LINKs can be viewed as a common denominator in both semblance hypothesis and LTP induction (has yet to be confirmed). Explanation of semblance formation through inter-postsynaptic membrane functional LINKs can fill the gaps in our findings of the correlation between memory and LTP and can explain why it has led to a large number of debates. One general argument is that any hypothesis of memory should be able to explain the relationship between LTP and the surrogate behavioral motor activity indicative of memory retrieval.
Previous experiments have shown that spatial learning becomes impaired after saturation of LTP (Moser et al., 1998). Later experiments have shown specific interrelationship between LTP and surrogate markers of memory retrieval (Whitlock et al., 2006). In this work it was shown that one-trial inhibitory avoidance learning in rats produced the same changes in hippocampal glutamate receptors as the induction of LTP with high-frequency stimulation. This study showed that learning-induced synaptic potentiation occludes high-frequency stimulation-induced LTP. Based on the findings in this work, a plausible explanation for the relationship between LTP and memory through the semblance hypothesis can be done as follows.
a. Learning first, followed by LTP induction:
According to the semblance hypothesis, prior learning events in a caged environment would have already made many islets of LINKed postsynaptic terminals (dendritic spines) in the hippocampi of the rats. Since associative learning opportunities are finite during caged life, we can expect a slow expansion (by LINKing more postsynaptic terminals with additional related learning events) of discrete islets of LINKed postsynaptic terminals as the rats grow up. When rats undergo avoidance learning (a novel instance of associative learning), we can expect the formation of functional LINKs between two or more islets of functional LINKs that are already present in the animal. Even though this is particularly important in this experimental context, it will also hold true in any novel associative learning.
In experiments using inhibitory avoidance testing (Whitlock et al., 2006), not all the recording electrodes recorded an increase in field excitatory postsynaptic potential (fEPSP) slope, indicating that ionic changes at the locations of the tips of these electrodes (CA1 dendritic tree) required to produce an increase in the fEPSP slope did not take place. However, among those electrodes that recorded an increase in fEPSP slope after inhibitory avoidance learning, a sufficient number of Shaffer-CA1 synapses were potentiated. Let I and II stand for two islets of functionally LINKed postsynaptic terminals that were already present in the animal before the avoidance learning session. During learning, it is likely that LINKs were formed between the islets (islets of LINKed postsynaptic terminals) I and II. This will generate a sudden increase in the size of an islet of LINKed postsynaptic terminals to nearly twofold, forming a mega-islet of LINKed postsynaptic terminals (Fig.14).
.
Figure 14. This illustration explains the basis of long term potentiation (LTP) based on the present hypothesis. The illustration shows potential LINKable site between islets of postsynaptic terminals (dendritic spines) (please see Fig.5 for details of the islets; they are visualized by the hypothetical cross-sectional view through functionally LINKed postsynaptic terminals) that belong to two different CA1 neurons. During an associative learning, LINK formed between the postsynaptic terminals (marked with asterisks) of islets 1 and 2 (large circles) can lead to the formation of a mega-islet that can continue to contribute to the LTP recorded from the recording electrode as explained in the text. The position of the stimulating electrode is at the Schaffer collaterals. Shaffer collaterals from the CA3 neurons synapse to the dendritic spines (postsynaptic terminals) of the CA1 neurons. Many of these postsynaptic terminals are functionally LINKed to form islets in an animal (see Figure 5 for details of the islets of functional LINKs). Here two such islets I and II (large circles) are shown. One of the postsynaptic terminals from each of the islets I and II is shown to continue towards the soma of the CA1 neurons. Activation of any one of the postsynaptic terminal within an islet will result in the EPSP spread towards the somas of the CA1 neuron. The islets are formed between postsynaptic terminals that are concurrently activated during previous associative learning. During an associative learning of a novel item or during induction of LTP (note the position of the stimulating electrode is at the Schaffer collaterals), a new functional LINK may form between the postsynaptic terminals (marked asterisks) of islets I and II. This can lead to the formation of a mega-islet combining the two islets. This can contribute to the LTP recorded from the recording electrode as explained in the text.
Activation of a postsynaptic terminal of this mega-islet of LINKed postsynaptic terminals can cause spread of depolarization between its postsynaptic terminals. Since a subset of postsynaptic terminals in the mega-islet already LINKed to one of the dendritic spines (postsynaptic membrane) on the dendritic tree of one CA1 neuron, multiple EPSPs from this subset will reach the main dendrite of a CA1 neuron simultaneously. This results in a summated EPSP at this dendritic location sufficient to produce a corresponding increase in current sink in the extracellular matrix. Immediately following the associative learning event, a proportion of sensory inputs reaching the animal for a long duration of time is likely to activate the postsynaptic terminals of this mega-islet, leading to prolonged activation of the main dendrites of the above CA1 neuron (until the CA1 neuron begins homeostatic mechanisms to reduce this prolonged and increased EPSP generation). The extracellular signal recorded from the apical dendrites of a population of pyramidal neurons in the stratum radiatum of the CA1 region in response to Schaffer collateral stimulation, namely the fEPSP, will now show an increase in amplitude and contribute to an increase in fEPSP slope for a long duration of time (LTP). This learning-induced LTP can occlude further LTP induction.
b. LTP induction first, followed by learning:
The occlusion process explained in the study (Whitlock et al., 2006) can be considered a bidirectional process, meaning that the induction of LTP in a sufficient number of synapses that are involved in inhibitory avoidance learning will prevent consequent avoidance learning. It is likely that hundreds of axons of the CA3 neurons in the Schaffer collateral pathway are activated by high-frequency stimulation (LTP induction), activating the postsynaptic terminals (dendritic spines) of a CA1 neuron. During this process, many postsynaptic terminals can get functionally LINKed due to the simultaneous activation of closely placed postsynaptic terminals by high-frequency stimulation (assuming that sufficient oxygenation state is present during this process). Some of these LINKs will occur between the islets of already LINKed postsynaptic terminals, leading to the generation of mega-islets. Following this, the activation of one or more postsynaptic terminal by a regular stimulus (not high frequency) can lead to the spread of depolarization between the postsynaptic terminals within the mega-islet. Since one or a small subset of postsynaptic terminals in the mega-islet originates from the dendritic tree of a single CA1 neuron, multiple EPSPs from these postsynaptic terminals can reach one dendrite of a CA1 neuron simultaneously. This results in an increase in the EPSP at these dendritic locations, leading to LTP. This artificially-induced LTP can occlude further learning-induced LTP.
If we can artificially induce LTP in a large number of fibers that includes those that are critical for the learning, then the animal may not be able to successfully retrieve specific memories after a new associative learning using those synapses following the LTP induction. This means that the animal cannot retrieve the specific memories; i. e., when a cue stimulus tries to retrieve a memory using these synapses, the induced depolarization spreads across all those postsynaptic terminals that are LINKed by the LTP induction. The retrieval using a specific cue now induces synaptic semblances at all those LINKed postsynaptic terminals in the mega-islet, some of which were LINKed non-specifically during LTP induction. Activation of those non-specific postsynaptic terminals will also lead to the activation of non-specific neurons, leading to the induction of non-specific network semblances that are not related to the learned item. In other words, the expected specificity of semblance for the learned item gets diluted by the large amount of non-specific semblances, preventing specific memory retrieval.
The following diagram (Fig.15) demonstrates the similarities between the cellular processes in LTP following induction and internal sensation of retrieved memory following associative learning.
.
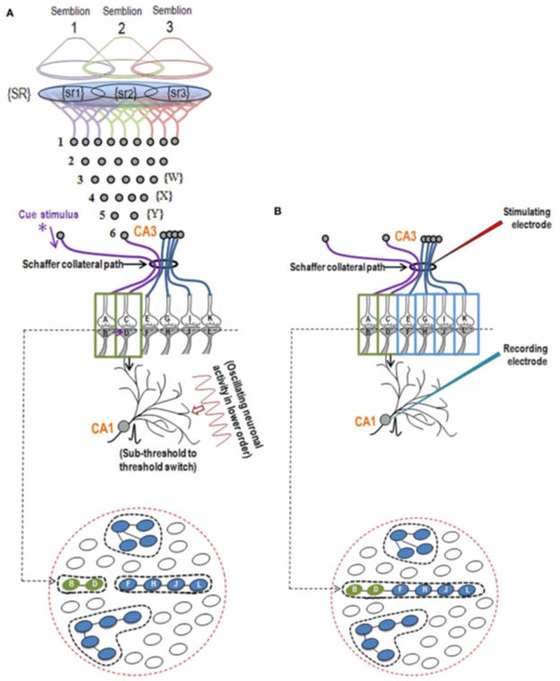
Figure 15. The illustration shows the structural mechanism of formation of internal sensation of memory and its relationship with a possible mechanism of LTP. A) During memory retrieval, a cue-stimulus reaching presynaptic terminal A depolarizes its postsynaptic terminal B, re-activates the hemi-fused inter-postsynaptic membrane and activates postsynaptic terminal D, evoking a cellular hallucination of arrival of sensory inputs at the latter's presynaptic terminal C. In normal conditions, an action potential reaches presynaptic terminal C when the CA3 neuron is activated. The sensory identity of the semblance of activity occurring at the postsynaptic terminal D consists of inputs from the set of neurons {Y} that synapse to the CA3 neuron. The set of neurons {Y} is normally activated by inputs from a set of lower order neurons {X}. The set of neurons {X} in turn is activated by a further large set of its lower order neurons {W}. Continuing this extrapolation toward the sensory level identifies a set of sensory receptors {SR}. {sr1}, {sr2}, and {sr3} are subsets of {SR} and are capable of independently activating the CA3 neuron. Hypothetical packets of sensory stimuli activating sensory receptor sets {sr1}, {sr2}, and {sr3} are called semblions 1, 2, and 3, respectively. The activation of the postsynaptic terminal D by the cue stimulus can lead to the virtual internal sensation of semblions 1, 2, 3 or an integral of them. A CA1 neuron (place cell in the context of spatial memory) is shown receiving sub-threshold excitatory postsynaptic potential (EPSP) from oscillating neuronal activities of its lower order neurons. Cue stimulus-induced activation of postsynaptic terminal D reaches the soma of its neuron in the CA1 region. If the CA1 neuron receives a baseline summated EPSP short of one EPSP to trigger an action potential, then the additional EPSP arriving from the postsynaptic terminal D can add to sub-threshold EPSP, inducing an action potential in the CA1 neuron, resulting in its concurrent activation during memory retrieval; this CA1 neuron will not otherwise be activated in the absence of prior associative learning. This can explain place cell (CA1neuron) firing occurring concurrently with spatial memory retrieval. Bottom Panel: Cross-section through the postsynaptic terminals showing a newly formed functionally LINKed postsynaptic terminals B and D during associative learning. Three other islets are also shown. B) Stimulation of the Schaffer collateral induces LTP by inducing postsynaptic membrane hemi-fusion between postsynaptic terminals that belong to islets of postsynaptic terminals B-D and F-H-J-L forming a mega-islet B-D-F-H-J-L. A regular stimulus at the stimulating electrode has now an increased probability of reaching the recording electrode through the large number of hemi-fused postsynaptic membranes within the large mega-islet, showing a potentiated effect when recorded from the CA1 neuron. Neuronal orders from 1 to 6 are numbered from the sensory receptors. Bottom Panel: Cross-section of an area containing the newly formed mega-islet of functionally LINKed postsynaptic terminals B-D-F-H-J-L formed during LTP induction. Two other islets are also shown. {SR}, Set of sensory receptors; {sr}, subset of sensory receptors. If LTP-induced mega-islets include postsynaptic terminals B and D, it reduces the specificity of retrieved memories in retrieving memories since the spread of activity through different non-specific postsynaptic terminals of the islet induce non-specific semblances (From Vadakkan, 2013).
The hypothesis has used one key assumption that internal sensation is induced at a specific location by a specific mechanism. Why should this be correct?
In order to build a hypothesis, some assumption has to be made in the beginning. If one assumption can consistently substantiate all the nervous system functions, then the probability for that assumption to be correct is high. This is similar to solving a system of linear equations having a unique solution. When only one variable remains unknown, then using its different relations with other variables the value of the unknown variable can be found mathematically. Alternatively, one is allowed to assign different values that unknown variable and use trial and error methods to solve the system. Similarly, in the case of a biological system where only one variable of internal sensation remains unknown, large number of known variables and their relationships with the unknown variable can be used by trial and error methods to solve the system. In deriving semblance hypothesis, induction of semblances as a system property was assumed to take place at the inter-LINKed postsynaptic terminal (dendritic spine) by the reactivation of the inter-postsynaptic functional LINK due to compelling reasons such as 1) some form of depolarization is always taking place at the postsynaptic terminal continuously, 2) the miniature EPSP generation cannot be blocked completely by any natural or synthetic chemicals on earth, 3) the formation of the inter-postsynaptic LINK can be achieved as a function of simultaneous activation of the abutted postsynaptic terminals during associative learning, 4) induction of semblance can then be derived as a function of lateral activation of the inter-postsynaptic LINK, 5) presence of different types of inter-postsynaptic functional LINKs having different life spans is suitable to explain how changes generated by learning can last for different durations, 6) it is possible to stabilize the functional LINK, providing ability to retain ability to retrieve memory of associatively learned items or events for different duration of time, 7) the lateral spread of activity through the inter-postsynaptic functional LINK contributes to the horizontal component of the oscillating potentials which is a requirement for inducing the system property of internal sensations, 8) semblance is a virtual property that suits to explain the virtual internal sensations of various higher brain functions, 9) semblance is a first-person property induced within the system towards which only the owner of the nervous system has access. All these fitting conditions make induction of semblance as an appropriate assumption. The possibilities for a spectrum of changes that can be formed during the generation of inter-postsynaptic LINK (see Fig.12) and their maintenance for a wide range of time periods shows the exact features that one would expect from the basic operational mechanism. Inter-postsynaptic functional LINK mechanism can operate in agreement with all the constraints offered by the findings listed in the Table 1 on the front page of this web site. Due to these reasons, the hypothesized mechanism is likely to be correct.
This work has explained induction of units of internal sensation of memory. How can it explain perception and consciousness?
Learning and memory were examined due to the advantages of examining them. Changes can be induced during learning and these changes are expected to be used to induce memories. Since these expected changes can be hypothesized, they can be tested to verify the hypothesis. This led to the derivation of inter-postsynaptic functional LINK formation and induction of units of internal sensations at the inter-LINKed spines as the basic operations. It is reasonable to expect that the basic mechanism of induction of units of internal has shared properties with the internal sensations of both perception and consciousness. Slight modification of the process of induction of units of internal sensations is expected to occur both during perception and in the operational mechanism of consciousness. In the case of perception, a real time process of induction of units of internal sensations has to be explained that can explain the large number of known properties of perception. This was carried out to explain visual perception (Vadakkan, 2015c). In the case of consciousness, it has to explain a) why a large number of units of internal sensations are getting induced while the animal is at rest, b) what is the net semblance formed by these units of internal sensations, and c) how it forms a background matrix upon which internal sensations in response to specific cue stimuli can be efficiently induced (Vadakkan, 2010).
What is the basic logic behind this work?
Let us imagine that there is a solvable system of linear equations. This means that there are few equations that contain several variables and these equations form a system, meaning that they are all interrelated. If we know the values of all the variables except one, then we will be able to find out the value of that unknown variable using simple mathematical method. But if we examine very carefully, we will see that the relationship of the unknown variable with the known variables within the equations can guide us to understand what the value of that unknown variable is. The above relationships are constraints that allow us to understand the value of the unknown variable. So, instead of using mathematical methods, we can also find the value of the unknown variable by trial and error method.
In a similar manner, the nervous system has a very large number of variables that are observed as different findings at various levels (molecular, cellular, inter-cellular, electrophysiological, systems, behavioral, and imaging). We already know all those findings and we have made large number of correlations between several findings already. Now we have one unknown variable, which is the generation of internal sensations within the mind. We are afraid to use it, since we don’t know how it is getting generated. Let us now use this variable in our findings. Here is an example. In addition to observing the behavior alone, let us include the fact that during memory retrieval there is an internal sensation of memory. Now, internal sensation is the only unknown variable within a large number of findings within the system. Now, we can use trial and error methods by using all the constraints offered by findings from different levels (Given in Table 2 on the Home page) to arrive at the mechanism of generation of internal sensations. The only difficulty is that we need to examine a very large number of observations from different levels to arrive at the solution and fine-tune it. Semblance hypothesis has used this method.
Are there any convincing evidence to show that this hypothesis is correct?
For a system that generates first-person internal sensations that are not accessible to third-person observers, it is necessary to use methods used in mathematics and physics to understand a phenomenon that is not sensible to our sensory systems. In this regard, the present work has derived a solution for the system using the underlying principle of finding a solution for a system of linear equations, which is also in agreement with the principle of unification. The resulted solution was used to triangulate (Munafò and Smith, 2018) findings from different levels. The derived solution is now is a position to explain the first-principle behind its operations.
Foremost, present hypothesis has viewed memories in their true sense as first-person internal sensations. The derived solution was found to have background properties for the induction of units of virtual internal sensations. The continuous depolarization of the spine heads (by both quantal release and EPSPs induced by intermittent arrival of action potentials at their presynaptic terminals by sensory stimuli from environment) sets the background state. Associative learning between two stimuli is expected to generate an inter-postsynaptic (inter-spine) LINK (IPL) between the spines that belong to two different neurons. In the above-explained background state, if one of the stimuli (cue stimulus) can incidentally reactivate the IPL, then it is expected to spark a cellular hallucination (semblance) at the inter-LINKed spine of receiving stimulus from the second stimulus. This matches with the expectation of a mechanism for cellular hallucination within the nervous system for explaining memory (Minsky, 1980). This is expected to provide a mechanism for the generation of virtual, first-person internal sensation of memory at physiological time-scales. We can make an extrapolation from the inter-LINKed spine towards the sensory receptors to identify the minimum sensory stimuli required to activate that inter-LINKed spine, which forms units of internal sensation. The combination of a) unique background state of the system, b) possibility for the formation of IPLs with mechanisms that can have different life spans, c) the suitability of the location of IPL that can allow the cue stimulus to reactivate it, d) observation of a suitable mechanism for the induction of virtual first-person internal sensation at physiological time-scales, and e) the perpendicular direction of propagation of potentials through the synapses and IPLs that can contribute vector components to the oscillating extracellular potentials whose frequency within a specific range controls the operation of the system matches with the expectations of a system that generates mind. This coincidence of multiple features provides the most convincing evidence.
Now, if we look at the above mechanism carefully, we can see that the ability to induce cellular hallucination (that constitutes first-person internal sensation) mandates that the dominant state of the system should be that the depolarization of the inter-LINKed spine head occurs with the arrival of activity from its presynaptic terminal. Therefore, lateral activation by the cue stimulus that depolarizes the inter-LINKed spine head from a lateral direction tricking the system to induce cellular hallucination at the inter-LINKed spine that constitutes memory should only occur for a limited duration of time. In other words, when the system keeps the arrival of depolarization from the presynaptic terminal as the dominant feature, then any incidental (or less duration) activation arriving through the IPLs will induce the cellular hallucination. If the ratio of the above two states is high, then the efficiency to trick the system to hallucinate in response to a lateral activation (which constitutes components memory) by a cue stimulus is easy. Since the quantal release that occurs all the time is at a saturated phase, then the system needs to control the duration of lateral activation. After a certain period of time, the system has to enter into a state of sleep (that prevents lateral activations by cue stimuli) following which the system resets itself back to the above dominant state. This provides an explanation for the substantive nature of sleep to the extent that the system will cease to function if it is not allowed to sleep for a few days. This provides another convincing evidence.
The above derived mechanism agrees with all the constraints offered by the very large number of observations from different levels (please refer to Table 2 in the Home page). Now, some of the convincing evidences that were found during continued examination of this hypothesis are the following. a) Most learning-induced changes will reverse back quickly as the animal moves through the environment, explaining working memory. The proportion of what remains is very less and they remain for varying periods of time for short- and long-term memories. In this regard, we expect that most of the learning-induced mechanism should be able to reverse back quickly. It should leave a small proportion of learning-induced changes to last for varying periods of time. So if we have arrived at the actual mechanism, then we should be able to observe changes to explain the above. By examining Figure 12 in this page, it can be seen that the formation of inter-postsynaptic functional LINKs by exclusion of water of hydration (Fig.12B) requires huge amount of energy and it reverses back quickly. Only a small proportion of these LINKs can form partial and complete hemifusions (Fig.12C,D) and will last for different periods of time. This forms a perfect fit with what we expect from learning-induced changes. This perfect fit shows that this mechanism is inevitable. b) As a continuation of what we found just now, there should be a mechanism for stabilization of learning-induced changes for a long period of time. Since the stage of complete hemifusion (Fig.12D) can be stabilized by different methods, it provides a suitable mechanism. In addition, when the newly formed inter-LINKed spines become part of an islet of inter-LINKed spines, it will be able to get both activated more frequently enabling its long-term maintenance. c) Furthermore, since dopamine cause spine enlargement (Fig.12E), it augments inter-postsynaptic functional LINK formation and its stabilization. This can result in retention of memories for a long period of time. This is another example for a perfect fit. d) Several correlations were found between the ability to learn and induce LTP. The derived mechanism has explained all those correlations and in addition explained some of the remaining uncorrelated observations in the field. This ability of the derived work provides further convincing evidence. e) Ability to provide a framework of a mechanism for perception, explaining various features of visual perception and finding a comparable circuitry for olfactory perception in a remote species Drosophila provides another convincing evidence. f) Ability to provide a framework for internal sensation of consciousness and how anesthetic agents can lead to loss of consciousness provides another evidence. g) Ability to explain a large number of common features of neurodegenerative disorders as a loss of function of the normal operational mechanism is another convincing evidence.
What are the key features of the testable circuit?
Functions of the brain include receiving sensory information, conscious interpretation of some of them, behavioral motor activities in response to them based on previous associative learning, and storing some of the newly received information. What type of a functional map of the nervous system can incorporate these functions? Why have we not succeeded in understanding the nervous system yet? What are we missing here? Once we understand the circuitry, it is necessary to explain a large number of functions within the system at different levels. What type of a circuit map can provide all these different types of observed features within the nervous system?
One of the essential features of the brain function is the formation of first-person inner sensations of higher brain functions (e.g. perception, memory, and consciousness) as a first-person property. Studying this property requires a completely new approach separate from current anatomical, molecular biological and electro-physiological approaches; but at the same time adhering to latter's basic principles. In order to understand what approaches need to be taken, it is required to build a hypothesis that can explain both first-person internal sensations and third-person findings from various levels. Since most of the higher brain functions are first-person internal sensations within the mind, the main emphasis while searching for the circuit properties should be given to explain it. In other words, the working hypothesis should have bridges from cellular and electro-physiological properties to that of the virtual internal sensation.
Once a hypothesis is built, it requires to be tested by three essential steps. First, test whether the hypothesis can explain what we have already discovered in various faculties of brain sciences. Luckily, the nervous system is complex enough that we can ask many questions to verify the validity of the hypothesis. Secondly, the new features in the hypothesis can be tested and its predictions can be verified. The third step is to address the issue of the first-person properties of brain functions. We need to carry out the gold standard test of replicating the mechanism in engineered systems to confirm the findings. This is necessary since the higher brain functions of the mind that are first-person properties require this step to convert internal sensations to appropriate read-outs so that we experimenters (third-persons) can understand them. Can we bypass this third step? The only alternative is theoretical. If the hypothesis can explain a very large number of disparate findings from multiple levels of the system, then this can be taken as sufficient evidence.
Semblance hypothesis was proposed towards achieving the above goals. It has provided explanations for various electro-physiological, behavioral and systems findings from different faculties of brain sciences. The essential feature of the hypothesis is the proposal of inter-postsynaptic functional LINK (IPL). An examination of the possible nature of this was then carried out. From examining disease processes that can alter the IPLs, it was possible to arrive at a reasonable conclusion that IPL is a candidate mechanism. This can be tested in animals and human samples. Its formation and functional role can be tested both during associative learning and its experimental correlate of the induction of long-term potentiation (LTP). By including IPLs and their functional role, we can obtain a new brain circuitry, which is explained in the following two figures (Fig.16).
.
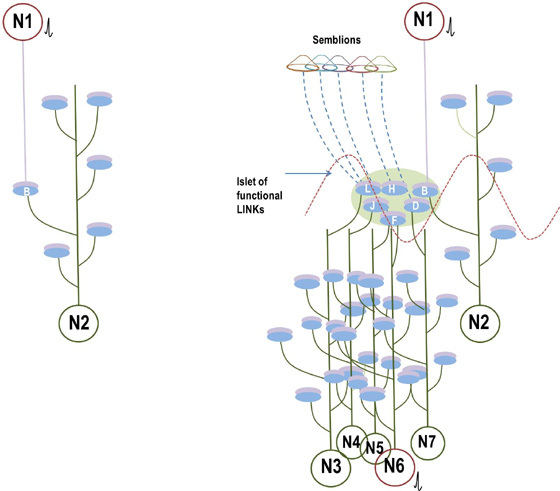
Figure 16. Comparison between the known synaptically-connected circuitry (left side) and the inter-postsynaptic functional LINK-mediated wiring(right side). Left panel: Synaptically connected conventional neuronal circuit diagram. There is one synaptic connection between neurons N1 and N2. The activation of neuron N1 induces an excitatory postsynaptic potential (EPSP) at postsynaptic membrane B. Provided neuron N2 is simultaneously receiving EPSPs from other neurons, the sum of which is just one EPSP short for spatial summation to trigger an action potential, then the EPSP arriving at postsynapse B from the activation of neuron N1 will lead to the firing of neuron N2. The contribution of the EPSP from the activation of Neuron N1 toward the temporal summation of EPSPs to elicit an action potential in neuron N2 should also be considered. Otherwise, a single EPSP or a train of few EPSPs reaching at postsynapse B alone may not induce an action potential of neuron N2. Right panel: Wiring diagram based on the present work. The activation of neuron N1 activates the inter-postsynaptic functional LINKs between the postsynapses in the islet of functional LINKs (see "Frequently asked questions" page on this website). The re-activation of postsynapse B that belongs to neuron N2 can provide EPSP and enable neuron N2 to fire an action potential similar to the threshold conditions explained for neuron N2 of the conventional wiring diagram (in the left panel figure). In addition, EPSPs spread to other hemi-fused postsynapses D, F, H, J, and L (depending on the extent of the spread through the islet) that can reach toward their neuronal somata. According to the supplementary rules, a total of six postsynapses are re-activated here, in comparison to only one by the canonical synaptic transmission (Figure in the left panel). This increases the probability of firing of sub-threshold activated neurons in the next order by bringing them to the threshold for activation. For example, neuron N6 continuously receives (n−1) EPSPs, just short of one EPSP toward either spatial or temporal summation to elicit an action potential. Arrival of the nth EPSP from the islet of functionally LINKed postsynapses enables neuron N6 to cross the threshold to elicit an action potential (shown in red). If neuron N6 is a motor neuron, it can evoke motor activity concurrent with the re-activation of the functionally LINKed postsynapses B, D, F, H, J, and L. Activity through these LINKed postsynapses will also evoke semblions for the formation of internal sensations provided these are located in regions of oscillatory neuronal activity. All the neurons in red receive sufficient summated EPSPs and fire action potentials. Note that the lateral spread of activity through the inter-postsynaptic functional LINKs provides the horizontal vector for the oscillatory neuronal activities observed both in the cortex and hippocampus. It is marked by a red wave passing through the islet of inter-postsynaptic functional LINKs. Even though ideally it should be drawn over the firing neurons, drawing it over the functional LINKs makes its operation more functionally directed (Modified from Vadakkan KI (2013) A supplementary circuit rule-set for neuronal wiring. Frontiers in Human Neuroscience 7:170.
The brain activity map that is built of the basic units presented here can explain the large number of nervous system functions (see Table 2 on the Home page) that are observed by different faculties of brain science. The basic cellular operating principle for the first person internal sensations of all the higher brain function should be sharing a common cellular mechanism. This will allow the common feature of induction of internal sensations in these higher brain functions. Inter-postsynaptic functional LINKs and the induction of semblance is capable of providing adequate mechanistic cellular features. Since the expected solution that can explain a wide range of findings at several levels is expected to be a unique one, the proposals made by semblance hypothesis can be considered for verification by testing the predictions made by the hypothesis and replicating the mechanism in engineered systems.
What are the implications of this work?
This work informs that the nervous system has two types of circuitry that operate in unison with each other. 1) A functional circuitry that operates through the formation, reactivation and reversal of IPLs. 2) A structural circuitry that operates through the synapses. Since firing of a single neuron can occur when it receives a fraction of its inputs (see section titled "What are the issues with studying neuronal firing (axonal spike) in understanding higher brain functions?" above), several neurons are kept at sub-threshold activation level under the influence of inhibitory neurons (Hangya et al., 2014; Karnani et al., 2014). In this context, the arrival of a small fraction of potentials through the IPLs will be able to fire some of those neurons for effective downstream end organ activation. Based on the IPL mechanism, internal sensations of various higher brain functions take place at the inter-neuronal inter-spine level. From the derivation of the mechanism, we have seen that integration of units of internal sensations induced at specific sets of inter-LINKed spines are essential for the generation of specific internal sensations associated with all the above higher brain functions. This is also essential to understand the internal sensations of various higher brain functions that include motivation, memory, perception, consciousness, aversion, reward, pleasure, anxiety, stress, fear, intentionality, hunger, thirst, and pain. By artificially altering neuronal firing at the neuronal orders at a level lower than that of the inter-LINKed spines, the internal sensations of most of the higher brain functions can be altered. But to understand the mechanism of generation of internal sensations of a higher brain function, we need to test the hypothesis of induction of units of internal sensations by the IPL mechanism and their integratation.
Why should this hypothesis be correct?
Science always seeks the truth. In order to understand certain phenomenon, it is necessary to examine it from multiple angles and make sure that our understanding is correct by all means. By keeping replication of the mechanism in an engineered system as the gold standard proof, the present work has kept the highest standard possible to undertake such an inquiry. Such an approach is expected to make one ask all possible questions in advance that will significantly reduce the probability of failure. The present work has used all the freedom it was necessary to put the pieces of the puzzle together.
When we say that we do not understand the brain, what we really meant are two things. a) We cannot inter-connect findings from different levels, and b) we do not understand how the first-person internal sensations in the mind are being generated. So our task is to find out the still undiscovered key operational mechanism for the generation of internal sensations that is also expected to inter-connect findings from different levels. In this context, we can use all the observations that we have already made from various levels and list the constraints offered by all of them (Given in Table 2 on the Home page). From those constraints, it is clear that the basic operational mechanism has to operate within the limited amount of freedom that it has. In other words, the constraints dictate what the solution is. The constraints offered by Nature are the guideposts that we can use to reach the solution. Wherever we reach, it should be the solution. So, we need to use all these constraints together and reach at the solution. Since a) the present work strictly adhered to these principles all throughout, b) large number of constraints were used to arrive at the solution, and c) large number of constraints were used to verify the solution, the derived solution is expected to be correct. The solution is expected to be a very unique one. At the same time, it is also expected to be a simple solution and universally present in all species of animals. The derived mechanism matches with these qualities. Now, we can further verify the hypothesis by asking more questions. Making attempts to disprove the hypothesis is part of this process. These can be continued until we become totally satisfied.
Give an example of a molecular evidence to suggest that this hypothesis must be correct?
I would like to quote the following words by Richard Feynman here. "When you have put a lot of ideas together to make an elaborate theory, you want to make sure, when explaining what it fits, that those things it fits are not just the things that gave you the idea for the theory; but that the finished theory makes something else come out right, in addition." This is an essential step to verify that we made a new discovery. If the semblance hypothesis is correct, then we should start observing new evidences that we never thought of while developing the hypothesis. I have explained many such examples. Here is a molecular example. In Figure 8, mechanism of IPL formation and maintenance is explained. IPL formation starts by interaction between the outer layer of the cell membranes of spines that belong to different neurons. This interaction can be stabilized by different mechanisms. If this is possible, then there should be molecular evidence to suggest such a mechanism is possible at suitable time-scales that matches with all the observed functions.
Membrane fusion is a high energy requiring process and the events have to overcome a high-energy barrier (Rand and Parsegian, 1984; Harrison, 2015). It is known that membrane fusion is facilitated by the action of certain specific intracellular proteins (Kozlovsky et al., 2004; Martens and McMahon, 2008). SNARE (soluble NSF (N-ethylmaleimide sensitive fusion protein) attachment protein receptor) proteins are known to provide energy for bringing together membranes until repulsive charges and overcome energy barrier related to curvature deformations during hemifusion between abutted membranes (Martens and McMahon, 2008; Oelkers et al., 2016). They also generate force to pull together abutted membranes as tightly as possible (Hernandez et al., 2012). By initiating the fusion process by supplying energy (Jahn and Scheller, 2006), SNARE proteins can lead to the formation of characteristic hemifusion intermediates (Lu et al., 2005; Liu et al., 2008). These properties of SNARE proteins highlight the functional significance of them to form hemifusion intermediates between the lateral spine head regions of spines. These intracellular factors can contribute to a short-lasting inter-spine membrane interaction leading to the generation of a short-lived IPL. Since mean inter-spine distance is more than mean spine head diameter (Konur et al., 2003), inter-neuronal inter-spine hemifusion is the best suitable type of inter-spine interaction.
SNARE proteins in the presynaptic terminals are largely studied. However, its presence within the dendritic spines shows that they have some functions here. All the properties of SNARE proteins described in the above paragraph show how it can contribute to the formation of hemifusion between the dendritic spines that belong to two different neurons. The specific property that SNARE proteins can form characteristic hemifusion intermediates (Lu et al., 2005; Liu et al., 2008) highlights the important functional property of this protein. Additional evidence includes the following. After the formation of IPLs in milliseconds of time during associative learning, they are expected to be reinforced by several delayed mechanisms. Since SNARE proteins are known to mediate fusion of intracellular vesicles containing AMPARs (a-amino-3-hydroxy-5-methyl-isoxazole propionic acid subtype of glutamate receptors) with the spine membrane (Lu et al., 2001; Kennedy et al., 2010), it forms a highly suitable mechanism that favor addition of membrane segments of the vesicles to the lateral spine head regions for the formation and maintenance of inter-spine hemifusion intermediates. In one previous study, it was found that fear conditioning drives AMPARs into postsynaptic regions of a large fraction of neurons in the lateral amygdala (Rumpel et al., 2005) after three hours. This study also found that memory is reduced if synaptic incorporation of AMPARs was blocked in as few as 10 to 20% of lateral amygdala neurons. Presence of AMPA GluR1 subunits at the lateral spine head region up to 25nm away from the synaptic cleft border (Jacob and Weinberg, 2014) indicates that this is the most probable region where AMPA GluR1 subunit vesicle exocytosis takes place. It also matches with the expectation of the location where inter-spine fusion can take place. These molecular events were not thought of at the time of derivation of this hypothesis. In fact, many of the findings were made only recently.
What are the odds of coming up with a correct hypothesis?
This was a question asked by one of my colleagues. I realize that this will also be a question arising in the minds of the readers. From a statistical point of view, the odds of coming up with a random hypothesis which is correct are very less. The reality is that I took hypothesis building as a hobby. I learned how to build a hypothesis and verify whether it can substantiate nervous system functions from different levels. I used to keep one hypothesis at a time. When I kept them for sufficient duration, I started finding weaknesses in them for providing sufficient logical rigor for a mechanism of nervous system functions. From each failure I learned a lesson, which helped me to build better ones. Interestingly, each of my subsequent hypotheses lived longer than the previous ones. Eventually, I reached my fifth hypothesis, which I kept for nearly a year before I abandoned it. It was based on charge transfer along the DNA molecules. I kept lot of hope & it made sense since neurons do not divide. But it failed in many aspects. Semblance hypothesis was the sixth hypothesis.
Was it possible to reach the details of the solution easily?
Even though the basic outline of the hypothesis and the concept of inter-postsynaptic functional LINK (IPL) became possible in early 2007, it took nearly four more years to identify the hidden mechanism that sparks the units of internal sensation at the IPL. Since the nervous system operates (for e.g. both learning and memory retrieval take place) only a narrow range of frequency of oscillating extracellular potentials, it is essential that the operational mechanism should have features to support this. Since the synaptic transmission in one direction and the propagation of depolarization across the IPLs that occurs in a perpendicular direction to this can provide the vector components to the oscillating extracellular potentials, it became very clear that the IPL mechanism is a highly suitable candidate. So I pursued to find a mechanism that can spark internal sensations at the inter-LINKed spine when a cue stimulus arrives. I was sure that I was "missing the trees for the forest" at that time. So my mind wandered around that location for months. When I published the article on consciousness in 2010, I was not aware the IPL mechanism. But, I was certain that a mechanism must be there. I was certain that it should be a simple mechanism since the system was evolved naturally. I was also certain that it will be capable of evading our attention so easily that I must search for it from different angles. So, I continued to search for a simple mechanism that can operate at time-scales of milliseconds. It was only by the end of 2010, I got the operational mechanism that can spark units of internal sensations. Immediately, I published the concept in early 2011 in the paper that explained "Processing semblances...". So, it took nearly four years. It was not easy! But it was worth pursuing!
What if this hypothesis is wrong?
This is a normal feeling following the development of any hypothesis. But if the hypothesis is correct, then the degree of this feeling will reduce with time. This is because there are a large number of ways by which the hypothesis can go wrong and there is only one unique way in which it can be correct. The major task of developing a hypothesis is to figure out the correct solution so that it can explain all the functions observed at various levels. In this approach, there are two major categories of mistakes that can occur and are the following. 1) Let us imagine that the nervous system consists of a large jigsaw puzzle in multiple dimensions and that we are trying to solve it using its parts. The most important thing is that we need to collect all the pieces of the puzzle from multiple levels and bring them on to the table. Since we will only solve for what we have on the table, missing some pieces from few levels can lead to a wrong hypothesis. 2) Secondly, let us imagine that we have brought all the pieces of the puzzle and they are of the same color (can be identified only by their shape, making the puzzle solving more difficult). So, direct matching of the pieces is the only way to confirm whether we are putting the pieces of the puzzle correctly. In this exercise, we may be able to put together a large number of pieces correctly, until we find that the remaining pieces won’t fit into the remaining slots (Hope every one of us had this experience). A good jigsaw puzzle will lead to these situations. Now, we need to dismantle everything and start building it again. Is this hypothesis reaching a stage described as above? Did it bring all the parts onto the table for assembling? Since this hypothesis has incorporated as many pieces of the puzzle as possible from multiple levels (by making sure that no non-redundant findings are left out) and was guided by the tight constraints offered by all those findings (given in Table 1 on the front page), these possibilities are expected to be very less. But, there is still a possibility that it is wrong. This is the reason why there is an open invitation to everyone for falsifying this hypothesis. When we make an observation that cannot be explained in terms of the present hypothesis, then we should consider it wrong. At that time, we should be able to build another hypothesis with more compelling explanations and inter-connectable features. It is also possible that someone can show that the present work is fundamentally wrong. But, it has not happened yet.
What is the motivation behind this work?
1. Nervous system generates first-person internal sensation in the mind, which is virtual in nature. These inner sensations then drive motor outputs in the form of speech and behavior that can be sensed by a third person. Examination of these third person findings alone is not sufficient to understand the system. It will need to acquire knowledge about a) how first-person internal sensations are induced, and b) how they are connected to the motor actions.
2. It is reasonable to expect that there is a solution. The reality is that we have difficulties in understanding it. Our minds can be trained to operate based on Bayes' rule. However, for solving difficult problems, we need to prepare our minds to go against all the priors. We can achieve this only by searching for totally new possibilities by ignoring all the priors. We need to look at the problem afresh similar to an outsider looking into the problem. It is a risky endeavor by all means. First, it will be very difficult to obtain funding to undertake such a work. Secondly, a work done in the absence of funding will be perceived as of low value. Thirdly, it has to face difficulties even from the very stage of publication. Is it worth taking the risk? Since we can use a large number of constraints from our previous findings to guide us towards finding the solution, we have a great opportunity to find the solution. If the constraints can guide us to reach a testable solution, then we should consider this as a potential solution and start taking steps to verify the predictions provided by the solution.
3. Let us imagine that we were able to derive a solution X. This solution should be in agreement with everything that we have already observed. If X can explain all the features of the system at various levels (in principle), then we should be prepared to accept its candidacy as a testable mechanism.
4. The virtual nature of internal sensations indicates that we should prepare ourselves to arrive at a first-principle that can provide testable predictions.
5. Based on the successful studies that allowed us to understand various phenomena in nature, the solution of the nervous system is expected to be a simple one. It is also expected to have features that continuously evaded our attention.
In order to solve the system (for all the levels), we need to use constraints obtained from multiple levels. This is a time consuming process that requires a large number of trial and error methods in the initial stage. In the absence of long-term unrestricted funding, it will not be possible to make serious attempts to solve the system. On the other hand, the large number of neurological and psychiatric patients needs urgent help. Without knowing the normal operating mechanism, it is not possible to design therapeutic methods for either prevention or treatment of several disorders. In fact, it is this context that motivated to undertake this work.
What is the main conclusion of the present work?
Semblance hypothesis has made a testable theoretical finding of a primary neuronal circuitry that resides within the synaptically-connected circuitry and has been evading our attention until now. This is the inter-postsynaptic functional LINK (IPL)-mediated circuitry described in the present work. In addition, the present work also derived a mechanism for the induction of units of internal sensation when IPLs are reactivated. According to the present work, the reactivation of IPLs provides vector components of the oscillating extracellular potentials whose frequency is tightly related to the efficiency of the system to induce internal sensations. An investigation to verify the presence of a spectrum of changes responsible for IPLs and the induction of internal sensations is needed.
What do we need to do in our experiments to verify the present work?
Current experiments are only examining single spines of neurons in to understand higher brain functions (by examining behavioral markers indicative of the generation of internal sensations of those brain functions. We find enlargement of spines and reduction in size or their elimination in experiments. We then start making correlations between these single spine changes with behavior. We fail to examine the interaction between the spine under examination and the spines in the neighboring region that belong to different neurons and how it can influence the internal sensation generated during those higher brain functions. If we look at any electron microscopic picture from cortical regions (for example, in this page see figure 9), the extracellular matrix space between abutted spines is very negligible. Since average inter-spine distance between spines on a dendritic branch is greater than the average spine diameter (Konur et al., 2003), the spines of a neuron are most likely abutted with spines that belong to other neurons. Undertaking studies by looking at how two abutted spines that belong to different neurons are interacting during a higher brain function such as learning at physiological time-scales of milliseconds will facilitate understanding of the inter-postsynaptic LINKs (IPLs) and their functions as explained in the present work. Only changes occurring at matching time-scales of learning or memory retrieval will be relevant. We cannot correlate any late occurring changes with the higher brain functions that generate internal sensations at time-scales of milliseconds.
Why do we need a first-person neuroscience?
Brain is an organ where first-person inner sensations of the mind are being generated. We have no previous experience in dealing with a system that generates first-person properties. It is a real challenge. If it is not for this challenge, many of us who work towards understanding the brain would not have been studying brain in the first place. We like to take these challenges, fail and fail again and eventually we hope one day we will become successful. In this context, we need to be very hard on the problem at hand and we need to face all the realities. Current third-person studies and efforts to use the results of such studies have shown us that we have to find the right tract. A typical example is news that is heartbreaking. During the last ten years, a large number of pharmaceutical companies moved away from drug development for neurological and psychiatric disorders (Wegener and Rujescu, 2013; Burke, 2014; Mehta et al., 2017). What is the reason? It is most likely that they have lost confidence in investing because many of the drug trials failed. For the community, it is a great loss since we have to live without having effective medications either to prevent or to reverse pathological changes in these disorders.
At this juncture, we are forced to ask, “Is there a possible reason why these drug trials constantly fail?” "What do we need to do?" A close examination shows that the studies of the higher brain functions and their dysfunctions are being carried out using surrogate markers such as speech output and behavioral motor actions for making conclusions. We are not attempting to understand the mechanism that operates to generate first-person inner sensations of memory. This is due to the lack of a scientific method to explore the first-person inner sensations of the higher brain functions. In order to design treatment methods to stop the loss of higher brain functions (e. g. memory problems) or alternations of higher brain functions (e.g., hallucinations), an exact science to explain a mechanism by which the first-person internal sensations are getting induced needs to be developed. Fixing the problems will become possible only when we truly understand the normal operational mechanism. In this context, it is necessary to develop specialized methods to explore and understand how first-person internal sensations are induced within the system. This can be best accomplished by having a dedicated first-person neuroscience. The main differences between current third-person neuroscience studies and a future first-person neuroscience studies are given the following table (Table 1).
| Third person neuroscience | First-person neuroscience |
Studies taking place at various levels are based on third-person observations. Examples include the following. Biochemical findings: Gene expression and action of protein molecules. Cellular changes: Outgrowths of neuronal processes, new neuron formation and their connections and neuronal firing Electrophysiological changes: Changes in AMPA and NMDA receptor currents, changes in postsynaptic potentials and changes in voltage-dependent calcium currents. System changes: Oscillating potentials recordable from using either surface or extracellular electrodes. Imaging findings: Changes in signals in fMRI, changes in neuronal ensembles that fire during a higher brain function. Behavioral changes: Speech and motor actions that can provide sensory inputs to third-person experimenters regarding the formation of first-person internal sensations. | First-person scientific approach deals with studying the mechanism of induction of first-person internal sensations. Currently these are considered as emergent properties. The apparent bottleneck in this approach is the access problem. What we need are methods and tools to overcome the challenges of the access problem. Since third-person experimenters cannot access the first-person properties, the methods to solve the issue involve the following critical steps. 1) Hypothesize a feasible mechanism that explains the nodal points at which internal sensation can emerge and specific conditions. It should have all the elements that can satisfy the requirements to explain findings made at various levels by different fields of neuroscience. 2) The hypothesized mechanism should be able to operate in union with the known circuit properties and should be able to explain various nervous system functions. 3) Using the hypothesized mechanism, develop a circuit to conduct the gold standard test of replicating the mechanism in engineered systems. At this stage, it is required to know the nodal points and conditions in which units of internal sensations emerge as a system property. 4) Devise methods to capture the emergent properties by converting them to suitable readouts for the third-person experimenters. This is a feasible step since we are designing the engineered system. |
Table 1. Key differences between third-person approaches that are being carried out currently and first-person approaches that can be carried out to understand the operation of the nervous system. | |