Recent Findings & New Explanations
Until now, there were no studies that examined the formation of IPLs. It is necessary to examine findings from different laboratories to examine whether they can be explained in terms of the present work.
A. In physiological conditions
Hotspots of dendritic spine turnover facilitate clustered spine addition and learning and memory. Frank AC, Huang S, Zhou M, Gdalyahu A, Kastellakis G, Silva TK, Lu E, Wen X, Poirazi P, Trachtenberg JT, Silva AJ. Nat Commun. 2018 Jan 29;9(1):422.
See supplementary figure 8. Learning leads to loss of spines and formation of new spines at those regions (spine turnover). Why would spines get lost. Based on semblance hypothesis (see figure 8 in the FAQ section of this website), learning leads to inter-neuronal inter-spine interaction leading to inter-postsynaptic functional LINKs (IPLs). Inter-spine fusion is at the extreme end of this spectrum of changes. The nature of IPLs depends on several factors. One of them is the type of fatty acids in the phospholipid molecules that form the spine membranes. If IPL formation leads to inter-neuronal inter-spine fusion, then it will lead to mixing of the contents of cytoplasm of two neurons. Since even adjacent neurons of a similar type varies in their protein content (Kamme et al., 2003; Cembrowski et al., 2016), it is reasonable to expect cellular mechanisms for closure of the fusion pore. If it is not possible, then the neurons will trigger mechanisms to remove the spines. This can explain spine loss. As a homeostatic mechanism, the involved neurons will generate new spines using phospholipids that resists inter-spine fusion. Thus, the basic operational mechanism of semblance hypothesis can be extended to provide a mechanistic explanation for spine turnover during learning.
References
Cembrowski MS, Bachman JL,
Wang L, Sugino K, Shields BC, Spruston N (2016) Spatial Gene-Expression
Gradients Underlie Prominent Heterogeneity of CA1 Pyramidal Neurons.
Neuron. 89(2):351-368.
Frank AC, Huang S,
Zhou M, Gdalyahu A, Kastellakis G, Silva TK, Lu E, Wen X, Poirazi P,
Trachtenberg JT, Silva AJ.
Hotspots of
dendritic spine turnover facilitate clustered spine addition and
learning and memory. Nat Commun. 2018 Jan 29;9(1):422
Kamme F, Salunga R, Yu J,
Tran DT, Zhu J, Luo L, Bittner A, Guo HQ, Miller N, Wan J, Erlander M
(2003) Single-cell microarray analysis in hippocampus CA1: demonstration
and validation of cellular heterogeneity. J Neurosci. 23(9):3607-3615.
Synapse-specific representation of the identity of overlapping memory engrams. Abdou K, Shehata M, Choko K, Nishizono H, Matsuo M, Muramatsu SI, Inokuchi K (2018) Science. 360(6394):1227-1231.
(will post soon)
Entorhinal cortex directs learning-related changes in CA1 representations (Grienberger and Magee, 2022) Nature. November, doi: 10.1038/s41586-022-05378-6
It is known that
Entorhinal cortex (EC) input to hippocampus (HP) is via two different
projections. 1) A trisynaptic path from EC layer II (ECII) to CA1
neurons via CA3 neurons and a monosynaptic pathway directly connecting
EC layer III (ECIII) to CA1 neurons (Fig.1).
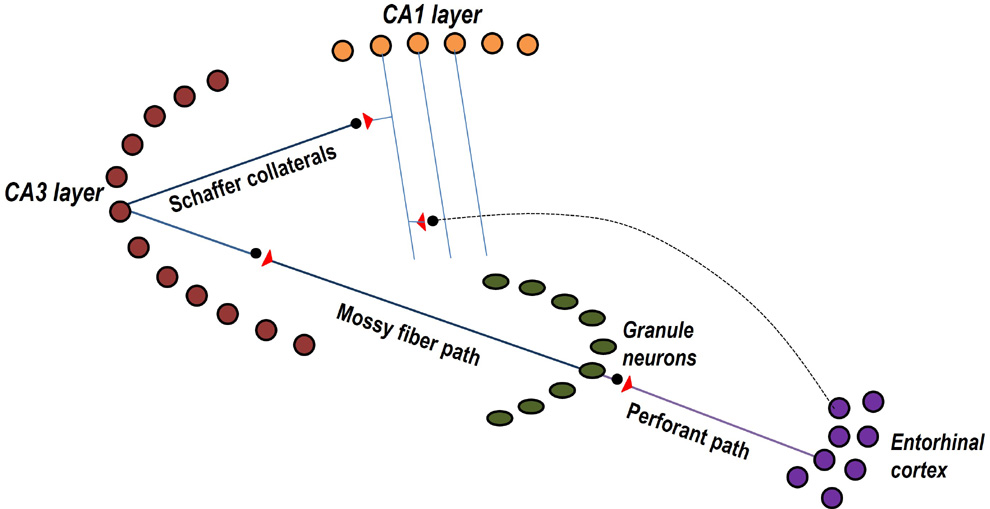
Figure 1.
Figure showing both trisynaptic and monosynaptic pathways between one
entorhinal cortical (EC) neurons and one CA1 pyramidal neuron.
Trisynaptic path from EC2 neurons connects through granule
neurons and neurons of the CA3 layer. Monosynaptic path from EC3 neurons
synapse to a CA1 neuron located at stratum lacunosum-moleculare layer at
apical tuft region of CA1 neuron.
CA1 pyramidal neurons that
fire somatic action potentials when the animal reaches a specific
location are called place cells (Moser et al.,
2015). One study has led to the
interference that EC3 to CA1 connections are involved in temporal
association memory (Suh et al, 2010).
Later, it was noticed that ability to associatively learn and memorize a
location in response to a cue stimulus is associated with increased
firing of CA1 neurons (Zhao et al., 2020)
as if there is an “overrepresentation” of these neurons to place memory.
It was also noticed that firing of CA1 neurons is associated with
long-term dendritic voltage signals initiated by inputs from EC3
sub-domain of EC (Magee and Grienberger, 2020).
Authors attribute this to occurrence of behavioral timescale synaptic
plasticity (BTSP) in the EC3-CA1 synapses. Recent experiments that
showed increased elevation of both EC3 activity and CA1 place field
density in response to a prominent reward-predictive cue stimulus in a
new environment led to the interpretation that EC directs
learning-related changes in CA1 representations (Grienberger
and Magee, 2022).
To provide a
mechanistic explanation for the above findings, it is necessary to
arrive at a mechanism that can provide explanations for the following
questions (Table 1).
1.
How can internal
sensation of a particular memory be explained?
2.
How can internal
sensation of a particular memory in response to a cue stimulus
explained?
3.
Since memory is associated with internal sensation of a conscious state
how can they both be explained in an interconnected manner?
4.
Explain
how the mechanism provides signals for a motor response at the same
time?
5.
Explain what learning-mechanism can lead to firing of set of CA1 neurons
(place cell firing or place field)?
6.
How to explain formation of long-duration dendritic voltage signals and
Ca2+ plateau potentials associated with learning changes in a
single trial (Takahashi and Magee, 2009;
Grienberger et al., 2014; Bittner et al., 2015)?
7.
Explain features of the mechanism that qualify it as an evolved
mechanism?
8.
Is it possible to explain various features exhibited by the system at
different levels of its operation?
Table 1.
Questions that a solution for the nervous system is expected to provide
answers for in an interconnected manner.
Challenges and features of a possible solution
Work by
Grienberger and Magee (Grienberger and Magee,
2022) infers that there are synaptic plasticity changes at the
EC3-CA1 synapses that lead to dendritic voltage signals in the dendrites
of CA1 neurons, which is associated with/in turn leads to firing of CA1
neurons. Increased firing of CA1 neurons is being interpreted as
“overrepresentation”. A mechanistic explanation is necessary to explain
both “plasticity” and “overrepresentation” with the type of clarity that
will allow its replication in engineered systems. Above requirements
given in Table 1 can be summarized to two questions. What
synaptic changes
inferred from
dendritic voltage signals, which are being referred to as synaptic
plasticity changes, can generate first-person inner sensation of memory?
How is the same mechanism linked to sudden firing of previously silent
CA1 neurons that made us to infer that they acquire place field
property?
Ability to retrieve memory
is currently being studied using surrogate markers such as behavioral
motor actions and speech. Instead of examining surrogate markers,
semblance hypothesis searched for a mechanism for first-person inner
sensations directly by asking the question, “At what location and by
what mechanism sparks first-person inner sensations?” Even though, third
person experimenter cannot sense or identify the formation for
first-person properties at this location, reaching a solution point
provides a mechanistic explanation for the most important function of
the nervous system. It can lead to finding methods to treat its
disorders and replicating the mechanism in engineered systems. This led
to derivation of semblance hypothesis (Vadakkan,
2007, 2013, 2019). It was based on the argument that if it
becomes possible to formulate a mechanism for generating internal
sensations that can also explain all features of the system exhibited in
different levels by an interconnectable mechanism, then the formulated
mechanism can be correct. It will be then possible to make testable
predictions that can be verified.
Explanation
Towards achieving this,
first a conditional definition for memory was made (Vadakkan, 2017).
This was followed by examining works that were carried out with an aim
to undertake the gold standard test of replicating the mechanism in
engineered systems, which will eventually lead to the development of
true artificial intelligence (AI). A pioneering work (Minsky,
1980) that viewed memories as hallucinations (internal sensations
of something in its absence) matched with the expectations of search for
a mechanism of first-person inner sensations. This laid a foundational
framework for a testable mechanism. Motivated by this, a search was
carried out in the nervous system to identify a change that can occur
during associative learning and can be used by one of the associatively
learned stimuli to generate hallucinations of second stimulus at the
time of memory retrieval.
In the background state,
head region of a dendritic spine (postsynaptic or input terminal) is
continuously getting depolarized by quantal release of neurotransmitter
molecules, in addition to occasional volleys of release of
neurotransmitter molecules when action potentials arrive at its
presynaptic terminal. Simultaneous activation of two abutted spines by
environmental stimuli is expected to form inter-postsynaptic
(inter-spine) functional LINK (IPL) during associative learning (Vadakkan,
2013), which forms the linchpin of derived mechanism. At the time
of memory retrieval, reactivation of this IPL by one of the
associatively learned stimuli leads to propagation of potentials to the
inter-LINKed spine previously activated by the second stimulus whose
memory is expected to get retrieved. In the background state of
continuous depolarization of spine head by quantal release of
neurotransmitter molecules, any sudden lateral activation of
inter-LINKed spine (in the absence of arrival of action potentials at
its presynaptic terminal) is expected to generate a hallucination that
the inter-LINKed spine is receiving a stimulus from the environment
through its presynaptic terminal. Qualia of first-person inner
sensations of a retrieved memory can be estimated by retrograde
extrapolation from the inter-LINKed spine towards identifying all the
sensory receptors (see figures 6 and 7 in FAQ section of this website).
A unit of semblance (semblion) is equivalent to minimum sensory stimuli
capable of stimulating a minimum subset of sensory receptors that will
stimulate the inter-LINKed spine. A natural retrograde extrapolation is
expected to occur at the time of memory retrieval as a system property
of systems where synaptic transmission and propagation of potentials
across the IPLs contribute intracellular potentials, whose corresponding
changes in the extracellular matrix (ECM) space form vector components
of oscillating extracellular potentials taking place within in a narrow
range of frequencies (Fig.2). This mechanism has provided
interconnected explanations for large number of findings in the system
and has generated several testable predictions (Vadakkan,
2019).
EC3 inputs to a CA1 neuron
synapse stratum lacunosum-moleculare at the dendritic apical tuft
region, whereas EC axonal terminals synapse with CA3 neurons whose
axonal terminals synapse with dendritic spines of CA1 neurons located in
the more proximal stratum radiatum (Fig.2).
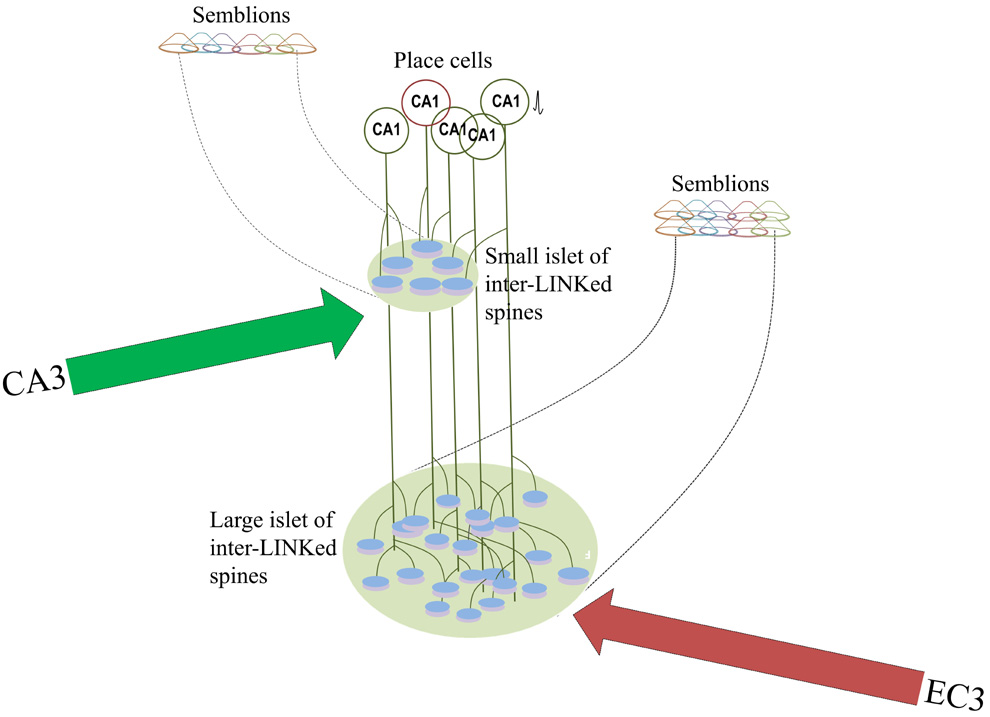
Figure 2.
Figure showing how trisynaptic and monosynaptic pathways from entorhinal
cortical (EC) neurons and a CA1 pyramidal neuron form islets of
inter-LINKed spines in the
stratum
lacunosum-moleculare and stratum radiatum layers respectively. Since
dendritic arbor at the apical tuft region where EC3 direct input arrives
is relatively bigger, it is reasonable to expect formation of large
number of IPLs at this location. This may explain an increased
horizontal component contributing to low frequency theta waveforms at
this location.
Oscillating
extracellular potentials show characteristic waveforms in these two
layers (Fernández-Ruiz et al., 2017)
reflecting the nature of spines that are involved in forming IPLs in
these locations. Both amplitude and frequency of these wave forms can be
explained in terms of vector components contributed by IPLs at these
locations. Low frequency theta waveforms can also be explained as the
net effect of the vector components contributed by IPLs in a larger
area. (see Fig. 1 in
https://www.cell.com/neuron/pdfExtended/S0896-6273(17)30101-0
and extracellular recordings:
https://www.nature.com/articles/nn.2894/figures/1).
Large number of interneurons are present in the L-M region (Capogna,
2011) indicates presence of IPLs between their spines and spines
of CA1 neurons modifying the qualia of internal sensations generated at
this location. The net potential generated at the islet of inter-LINKed
spines propagates to the axon hillock of the CA1 neurons, allowing some
of the sub-threshold activated CA1 neurons to fire somatic action
potential.
Firing of EC3
neurons followed by firing of CA1 neurons prompt one to infer
involvement of EC3-CA1 synaptic changes. Inhibition of NMDA receptor
channels at these synapses cause inhibition of both learning and memory
retrieval. The involvement of synapses towards the formation and
reactivation of IPLs can be interpreted as synaptic plasticity changes
involving EC3-CA1 synapses. Since IPL mechanism can explain generation
of inner sensations can occur concurrent with firing of CA1 neurons, it
can be viewed as a better explanation. Large amplitude synaptic inputs
delivered by EC3 axons in apical dendritic tree of CA1 (Megias
et al., 2001; Steward and Scoville, 1976) can lead to the
formation of IPLs between spines of different CA1 neurons located in the
stratum lacunosum-moleculare layer at apical tuft region of CA1 neuron.
Both increased
probability and duration of plateau
potentials (Takahashi and Magee, 2009; Bittner
et al., 2015) can be explained in terms of propagation of
potentials through islets of inter-LINKed spines formed by large number
of abutted spines stimulated. IPL reactivation provides additional
potentials to sub-threshold activated CA1 neurons allowing them to fire
an action potential.
Place cells are
CA1 pyramidal neurons that fire a somatic action potential (somatic
spike). Using CA1 neurons that fire, hippocampal maps were created to
study their association for spatial memory performance (Dupppret
et al., 2010), which led to the inference is that accumulation of
place fields is responsible for spatial learning. A pyramidal neuron
that has thousands of input terminals can fire a somatic action
potential when nearly 140 inputs signals arrive at any combination of
input terminals (Eyal et al., 2018).
Extreme degeneracy of input signals in firing a CA1 neuron makes firing
of a CA1 neuron non-specific with respect to the location from where
potentials arrive. Furthermore, large plateau potential anticipated to
be generated by islet of inter-LINKed spines leads to more
non-specificity of CA1 neuronal firing with respect to the input
signals. Inhibition of CA1 firing by AP5
(antagonize NMDA receptors at the synapses of spines of CA1 neuron) or
inhibitor of plateau firing CAV2-3 channel blocker SNX-482 occur due to
inhibition of neurons. Since synapses are necessary for IPL
mechanism to operate, chemicals that block synaptic functions will stop
cognitive function and CA1 neuronal firing (place cell firing).
Conclusion
Moving away from making
presupposition that a single neuron process information, basic questions
were asked to obtain a mechanistic explanation. The solution for the
system should be able to explain how first-person property of inner
sensation of a place occur in the nervous system along with remaining
features such as 1) optional concurrent behavioral motor actions, and 2)
firing of CA1 neurons. Interaction between spines of different neurons
separated by narrow extracellular matrix provides a solution for the
system. EC3 inputs synapse with spines of CA1 neuron in the stratum
lacunosum-moleculare layer at the dendritic apical tuft region. Since
spines of adjacent pyramidal neurons overlap with each other,
interaction between these spines is expected to occur to generate inner
sensations. When these interactions contribute to additional potentials
to a subthreshold activated pyramidal neuron, it fires a somatic action
potential, which is being viewed as place cells.
The observation
that CA1 firing is inhibited by inhibitor of plateau firing Cav2.3
Ca2+channel blocker indicates that it is capable of blocking
somewhere along the route from EC3 to CA1. Explanations for different
phenomena indicated presence of IPLs between spines of different CA1
neurons. For example, Cav2.3 Ca2+
channels mediate epileptiform activity such as afterdepolarization,
plateau potentials and exacerbation of low-threshold Ca2+
spikes resulting in seizure initiation and propagation (Wormuth
et al., 2016). It was shown how IPL mechanism can explain seizure
generation (Vadakkan, 2016). Cav2.3
Ca2+ channels in the presynaptic terminals are involved in
LTP (Breustedt et al., 2003). It was
previously explained how synapses are involved in IPL formation and
reactivation to influence LTP (Vadakkan, 2019).
Furthermore, Cav2.3 Ca2+
channels have a highly organized spatial distribution with predominant
expression in the proximal or distal dendrites (Westenbroek
et al., 1995).
The IPL mechanism provided
by semblance hypothesis is able to explain how “engram neurons” that are
often seen as “representations” of memory and “synaptic plasticity”
changes. Conducting experiments to study interaction between abutted
spines that belong to different neurons by the arrival of two
associatively learned stimuli can be used to verify the presence of
IPLs. GFP-reconstitution method across synaptic partners (GRASP) (Feinberg
et al., 2008; Gordon and Scott, 2009; Fan et al., 2013; Macpherson et
al., 2015; Shearin et al., 2018) was used to study synaptic
connections. Trans-Tango (Talay et al., 2017)
and TRACT (Huang et al., 2017) were
invented for anterograde trans-synaptic tracing. Retrograde
trans-synaptic tracing was carried out using a method called BAcTrace, (Cachero
et al., 2020). Recently, retro-Tango method of retrograde
synaptic tracing was developed (Sorkaç et al.,
2022). Since TRACT, trans-Tango and retro-Tango methods allow
neurons to show synaptic partners, similar approaches can be utilized to
develop inter-spine tracers to study IPL links between spines that
belong to different neurons of one neuronal order. Explanations provided
here are expected to provide motivation to verify interaction between
spines that belong to different neurons.
References
Bittner KC, Grienberger C, Vaidya SP, Milstein AD, Macklin JJ, Suh J,
Tonegawa S, Magee JC (2015) Conjunctive
input processing drives feature selectivity in hippocampal CA1 neurons.
Nat. Neurosci. 18:1133–1142.
PubMed
Breustedt J, Vogt KE, Miller RJ, Nicoll RA, Schmitz D (2013)
Alpha1E-containing Ca2+ channels are involved in synaptic plasticity.
Proc. Natl. Acad. Sci. USA. 100(21):12450–12455.
Cachero S, Gkantia M, Bates AS, Frechter S, Blackie L, McCarthy A,
Sutcliffe B, Strano A, Aso Y, Jefferis G (2020) BAcTrace, a tool for
retrograde tracing of neuronal circuits in Drosophila. Nat. Methods,
17(12):1254–1261.
https://doi.org/10.1038/s41592-020-00989-1
Capogna M (2011) Neurogliaform cells and other interneurons of stratum
lacunosum-moleculare gate entorhinal-hippocampal dialogue. J.
Physiol. 589(Pt 8):1875–1883. doi:
10.1113/jphysiol.2010.201004.
Fan P, Manoli DS, Ahmed OM, Chen Y, Agarwal N, Kwong S, Cai AG, Neitz J,
Renslo A, Baker BS, Shah NM (2013) Genetic and neural mechanisms that
inhibit Drosophila from mating with other species. Cell.
154(1):89–102.
https://doi.org/10.1016/j.cell.2013.06.008
Feinberg EH, Vanhoven MK, Bendesky A, Wang G., Fetter RD, Shen K,
Bargmann CI (2008) GFP reconstitution across synaptic partners (GRASP)
defines cell contacts and synapses in living nervous systems. Neuron.
57(3):353–363. https://doi.org/10.1016/j.neuron.2007.11.030
Fernández-Ruiz A, Oliva A, Nagy GA, Maurer AP, Berényi A, Buzsáki G
(2017) Entorhinal-CA3 dual-input control of spike timing in the
hippocampus by theta-gamma coupling. Neuron. 93(5):1213–1226.e5. doi:
10.1016/j.neuron.2017.02.017.
Gordon MD, Scott K (2009) Motor control in a Drosophila taste circuit.
Neuron. 61(3):373–384. https://doi.org/10.1016/j.neuron.2008.12.033
Grienberger C, Chen X, Konnerth A (2014) NMDA receptor-dependent
multidendrite Ca2+ spikes required for hippocampal burst firing in vivo.
Neuron. 81:1274–1281.
PubMed
Hollup SA, Molden S, Donnett JG, Moser MB, Moser EI (2001) Accumulation
of hippocampal place fields at the goal location in an annular watermaze
task. J. Neurosci. 21:1635–1644.
https://doi.org/10.1016/j.neuron.2017.10.011
Huang TH, Niesman P, Arasu D, Lee D, De La Cruz AL, Callejas A, Hong EJ,
Lois C (2017) Tracing neuronal circuits in transgenic animals by
transneuronal control of transcription (TRACT). Elife. 6.664.
https://doi.org/10.7554/eLife.32027
Macpherso LJ, Zaharieva EE, Kearney PJ, Alpert MH, Lin TY, Turan Z, Lee
CH, Gallio M (2015) Dynamic labelling of neural connections in multiple
colours by trans-synaptic fluorescence complementation. Nat. Commun.
6:10024. https://doi.org/10.1038/ncomms10024
Magee JC, Grienberger C (2020) Synaptic plasticity forms and functions.
Annu. Rev. Neurosci. 43:95–117. PubMed
Megias M, Emri Z, Freund TF, Gulyas AI (2001) Total number and
distribution of inhibitory and excitatory synapses on hippocampal CA1
pyramidal cells. Neuroscience. 102:527–540. PubMed
Moser MB, Rowland DC, Moser EI (2015) Place cells, grid cells, and
memory. Cold Spring Harb Perspect Biol. 7(2):a021808.
PubMed
Shearin HK, Quinn CD, Mackin RD, Macdonald IS, Stowers RS (2018)
t-GRASP, a targeted GRASP for assessing neuronal connectivity. J.
Neurosci. Methods. 306:94–102.
https://doi.org/10.1016/j.jneumeth.2018.05.014
Sorkaç A, Moșneanu RA, Crown AM, Doruk Savaş D, Okoro AM, Talay M, Barnearetro G (2022) retro-Tango enables versatile retrograde circuit tracing in Drosophila. Biorxiv. https://doi.org/10.1101/2022.11.24.517859
Steward O, Scoville SA (1976) Cells of origin of entorhinal cortical
afferents to the hippocampus and fascia dentata of the rat. J. Comp.
Neurol. 169:347–370. PubMed
Suh J, Rivest AJ, Nakashiba T, Tominaga T, Tonegawa S (2011) Entorhinal
cortex layer III input to the hippocampus is crucial for temporal
association memory. Science. 334:1415–1420. PubMed
Takahashi H, Magee JC (2009) Pathway interactions and synaptic
plasticity in the dendritic tuft regions of CA1 pyramidal neurons.
Neuron. 62:102–111. PubMed
Talay M, Richman EB, Snell NJ, Hartmann GG, Fisher JD, Sorkac A, Santoyo
JF, Chou-Freed C, Nair N, Johnson M, Szymanski JR, Barnea G (2017)
Transsynaptic mapping of second-order taste neurons in flies by
trans-Tango. Neuron. 96(4):783–795 e784.762. PubMed
Vadakkan KI (2007) Semblance of activity at the shared post-synapses and
extracellular matrices - A structure function hypothesis of memory.
ISBN:978-0-5954-7002-0
Vadakkan KI (2013) A supplementary circuit rule-set for the neuronal
wiring. Frontiers in Human Neuroscience. 1;7:170.
PubMed
Vadakkan KI (2019) A potential mechanism for first-person internal
sensation of memory provides evidence for the relationship between
learning and LTP induction. Behav Brain Res. 360:16-35.
Vadakkan KI (2019) From cells to sensations: A window to the physics of
mind. Phys Life Rev. 31:44-78.
PubMed
Westenbroek RE, Sakurai T, Elliott EM, Hell JW, Starr TV, Snutch TP,
Catterall WA (1995) Immunochemical identification and subcellular
distribution of the alpha 1A subunits of brain calcium channels. J.
Neurosci. 15(10):6403–6418.
Wormuth C, Lundt A, Henseler C, Müller R, Broich K, Papazoglou A,
Weiergräber M (2016) Review: Cav2.3 R-type voltage-gated Ca2+
channels - Functional implications in convulsive and non-convulsive
seizure activity. Open Neurol J. 10:99-126.
Zhao X, Wang Y, Spruston N, Magee JC (2020) Membrane potential dynamics
underlying context-dependent sensory responses in the hippocampus.
Nat. Neurosci. 23(7):881-891.
PubMed
Invariant stimulus decoding using correlated neuronal fluctuations
(Ebrahimi et al., (2022) Nature. May 605(7911):713-721.
Current studies in
neuroscience are carried out by examining neuronal firing as a unitary
property of the nervous system. Patterns of neuronal firing called
neural population codes are used to make correlations with different
brain functions. It is also used to make representations of sensory
perception using animal behavior in response to sensory stimuli. By
analyzing neuronal firing in response to a specific stimulus over
different timescales, it is found that variations of elements (neurons
that fire) occur within each set of neurons that fire (Rumyantsev et
al., 2020; Driscoll et al., 2017; Montijn et al., 2016). A recent study
(Ebrahimi et al., 2022) shows occurrence of a) sensory coding redundancy
near the beginning of perception of a sensory stimulus, and b) shared
co-fluctuations of neuronal firing in different areas of brain.
To decode
behavioral response and to progress from representation to causation, it
is necessary to understand a mechanistic explanation how first-person
property of sensory perception is generated and how it is associated
with firing of different sets of neurons. Since sensory perception
occurs in a conscious mind, it is necessary to examine how first-person
properties occur within the nervous system and how this mechanism is
correlated with the third person observation such as firing of neurons.
Studies have shown that oscillating extracellular potentials need to be
maintained in a narrow range for conscious perception. Oscillating
extracellular potentials is a reflection of ionic changes occurring
across neuronal cellular membranes that in turn reflect the nature of
propagation of potentials across the neuronal processes. Since
oscillations across three-dimensional space of extracellular matrix
(ECM) can only be explained by the occurrence of vector components that
contribute to these oscillations, it is necessary to find mechanisms
that lead to generation of these vector components. Since there are
oscillations of potentials with different amplitudes and frequencies in
space, it is also necessary to explain how and where the vector
components contributing to these oscillations occur. This also provides
an opportunity to hypothesize mechanism/s that can lead towards a
solution for the system that can explain how first-person properties are
formed within the system.
Since inner sensations of memories are
first-person properties, it is possible to ask, “What type of a change
should occur within the system during associative learning that can be
used to generate first-person inner sensations of retrieved memories?”
Once it becomes possible to generate a hypothesis for such a mechanism,
it allows us to test for the occurrence of a change during learning.
With this aim, semblance hypothesis synthesized a general framework of a
mechanism (Vadakkan, 2007). When attempts are made to generate
artificial intelligence by transferring mechanism of natural
intelligence to engineered systems, it becomes necessary to understand
how first-person properties are generated within the system. Towards
this attempt, memories were viewed as hallucinations (inner sensation of
a sensory stimulus in its absence) and a framework for a mechanism was
developed (Minsky, 1980). When semblance hypothesis was further examined
in line with K-lines proposed by Marvin Minsky, it was possible to
derive formation of inter-postsynaptic functional LINK (IPL) as linchpin
change occurring during learning whose reactivation is expected to
generate internal sensation of memory (Vadakkan, 2013). Accordingly,
reactivation of IPLs from a lateral direction by a specific cue stimulus
is capable of generating units of inner sensations. Propagation of
potentials through established IPLs provides one of the vector
components to oscillating extracellular potentials at the locations
where postsynaptic terminals of the same neuronal order interact with
each other in their orthogonal organization with respect to linear
orientation of neurons in the consecutive neuronal orders. Synaptic
transmission between linearly-oriented neurons of different neuronal
orders provide the second vector component perpendicular to that occur
through the IPLs.
Many neurons are held at subthreshold
activation states. Background subthreshold activation of a neuron
depends on natural environmental stimuli (for example, gravity), phase
of oscillation of oscillating extracellular potentials and spatial and
temporal summations of potentials. Using propagation of potentials that
contribute vector components, it is possible to explain both generation
of oscillating extracellular potentials and addition of potentials to
several neurons that are held at subthreshold activation levels. In
other words, stimulus under investigation provides additional EPSPs to
different sets of neurons that are being held at subthreshold activation
states and allows them to fire action potentials. This is demonstrated
in Figure 1.
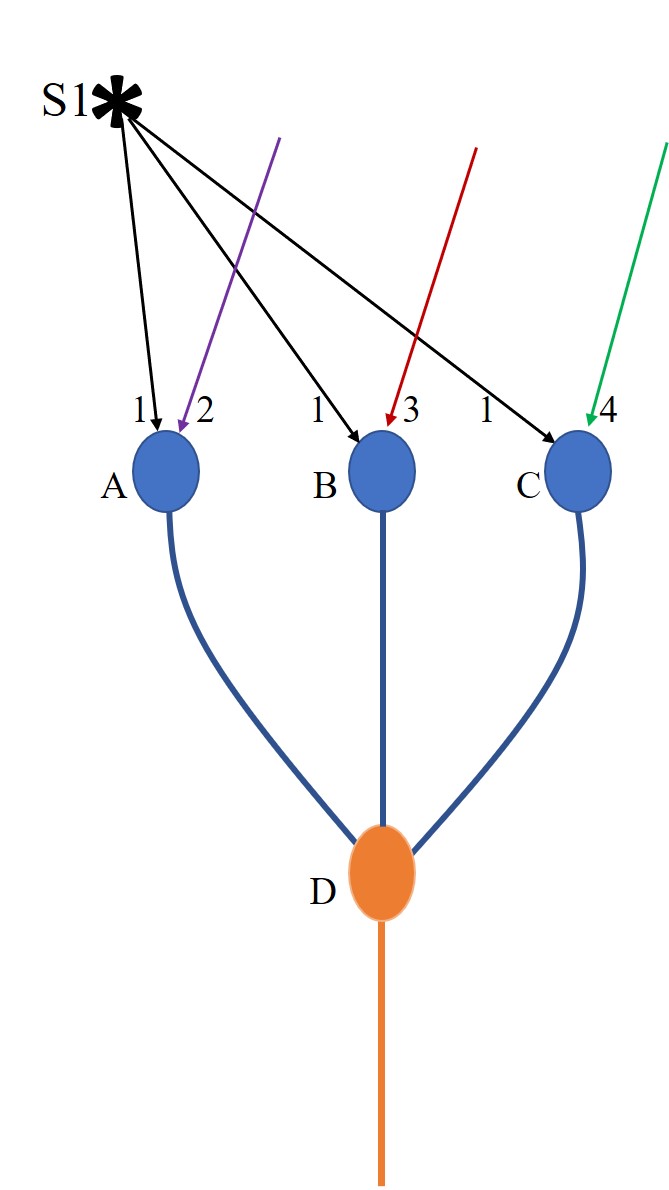
Figure.1.
Sensory coding redundancy explained using an example. When a stimulus
arrives, it will provide sufficient stimulus to several first order
neurons that leads to their firing. Action potential triggered by an
excitatory neuron will lead to synaptic transmission at the synapses on
all its axonal terminals. The EPSP generated at a postsynaptic terminal
gets spatially or temporally summated with the rest of the ESPSs
arriving at the axonal hillock. Depending on whether the net summated
EPSP crosses the threshold for firing, the postsynaptic neuron either
fires or does not fire. This is pictorially depicted by the example of
three neurons A, B and C that receive sub-threshold activations short of
two EPSPs at their baseline resting states. A specific stimulus under
investigation is marked “S1”. It provides EPSPS to all three neurons A,
B and C. EPSPs 2, 3 and 4 reaching neurons A, B and C respectively
arrive from either internal or external stimulus at the same time. If
neuron A receives EPSP 1 and 2 simultaneously, it will lead to its
firing.
If neuron B receives EPSP 1 and 3
simultaneously, it will lead to its firing.
If neuron C receives EPSP 1 and 4
simultaneously, it will lead to its firing. Hence, when EPSP1 arrives,
firing of neurons A, B and C depends on whether they are receiving
additional EPSPs concurrently or temporally so that these neurons fire.
If D is a neuron of the second order of neurons and if it is being held
at subthreshold state short of one EPSP and if it has inputs from
neurons A, B, and C, then firing of either one of the neurons A or B or
C will cause its firing. Hence, a stimulus under examination can cause
firing of sets of A and D or A, B and D or A, B, C and D or B and D or
B, C, and D or C and D, or A, C and D simultaneously.
Second explanation is needed for the
observation of sensory coding redundancy at the start of perception
(Ebrahimi et al., 2022). Redundancy of inputs is expected to minimize
the effect of variations in the sets of neurons that fire. But the
stochastic nature informs that something new is taking place within the
circuitry. This provides a unique opportunity to examine any proposed
hypothesis of brain functions for its explanatory capabilities. Since
any set of nearly 140 input signals arriving through nearly tens of
thousands of input terminals of a pyramidal neuron in the cortex can
fire a neuron (Palmer et al. 2014; Eyal et al., 2018), there is presence
of extreme degeneracy of input signals in firing a neuron (Vadakkan,
2019). Since many neurons are being held at subthreshold activation
levels in the background state, and since there is presence of
continuously varying internal stimuli originating from within the
system, arrival of different combinations of input signals can lead to
firing of the same neuron. By extension, it I possible to infer that
stimulus from a sensory stimulus under examination can generate
potentials that can reach neurons where they get summated with
potentials from a) input signals generated either internally or
externally, and b) reactivation of IPLs that also contribute to
oscillating extracellular potentials. As the
interval between testing increases, occurrence of different associative
learning events will add more IPLs to the system. In addition, some IPLs
will get reversed back over time. These can lead to changes in the net
EPSPs arriving at the axonal hillocks of neurons that are held at
subthreshold activation states. This can explain variations of neuronal
sets that fire in response to a specific stimulus over different
timescales (Rumyantsev et al., 2020;
Driscoll
et al., 2017;
Montijn et al.,
2016).
Thirdly, it is necessary to explain how
different areas of brain show shared co-fluctuations of neuronal firing.
As explained in the previous paragraph, addition and deletion of IPLs
over time will lead to changes in the sets of neurons that fire. Both
propagation of potentials along projection neurons between different
brain areas, and maintenance of both frequency and amplitude of
waveforms of oscillating extracellular potentials are expected to allow
maintenance of correlated subthreshold states of sets of neurons at two
locations. When a stimulus arrives, this can provide inputs to
subthreshold-activated neurons at those two locations and allow them to
cross thresholds for firing.
Both
correlated fluctuations and visual coding redundancy that are
time-varying throughout stimulus presentation rise within 100ms and peak
around 200ms after sensory stimulus onset (Ebrahimi
et al., 2022). This time delay matches with the time needed for
expansion of several spines that eventually leads the formation of more
IPLs. These late-forming IPLs have no role in early perception. However,
their physiological utility is in maintaining continuity of perception
of a stimulus. The inference made in the work that some neurons have
greater intrinsic variability in the fidelity of stimulus encoding than
others can be explained by 1) Different combinations of inputs add
potentials that will allow the summated EPSPs to cross the threshold to
fire a neuron, and 2) IPLs are formed in excess such that same semblance
can be generated from different combinations of units of semblance
generated at those IPLs. Inference from all the observations tempted the
authors to speculate for presence of “non-interfering communication
channels” in the neocortex, which can be explained in terms of the IPL
mechanism.
Driscoll LN, Pettit, NL, Minderer M, Chettih SN, Harvey CD (2017)
Dynamic reorganization of neuronal activity patterns in parietal cortex.
Cell 170:986-999.
Ebrahimi S, Lecoq J, Rumyantsev O, Tasci T, Zhang Y, Irimia C, Li J,
Ganguli S, Schnitzer MJ (2022) Emergent reliability in sensory cortical
coding and inter-area communication. Nature 605(7911):713-721.
Eyal G, Verhoog MB, Testa-Silva G, Deitcher Y, Benavides-Piccione R,
DeFelipe J, de Kock CPJ, Mansvelder HD, Segev I (2018)
Human cortical pyramidal neurons: From spines to spikes via models.
Front. Cell Neurosci.
12:181.
PubMed
Montijn JS, Meijer GT, Lansink CS, Pennartz CM (2016) Population-level
neural codes are robust to single-neuron variability from a
multidimensional coding perspective. Cell Rep. 16:2486-2498.
Palmer LM, Shai AS, Reeve JE, Anderson HL, Paulsen O, Larkum ME (2014)
NMDA spikes enhance action potential generation during sensory input.
Nat. Neurosci. 17(3):383-390
PubMed
Rumyantsev OI, Lecoq JA, Hernandez O, Zhang Y, Savall J, Chrapkiewicz R,
Li J, Zeng H, Ganguli S, Schnitzer MJ (2020) Fundamental bounds on the
fidelity of sensory cortical coding. Nature 580:100-105.
PubMed
Activation of a specific glomerulus by human odour in Aedes mosquitos
Human odor stimulus leads to
activation of a specific glomerulus in Aedes mosquitos. Every
glomerulus receives more than on sensory neuronal input. For example, in
Drosophila melanogaster a single glomerulus that senses CO2
has more than one sensory neuron arriving to that glomerulus
(Jones et al., 2007). Close examination of the findings in a
recent work (Zhao et al., 2022) shows
that more than one sensory neuron is necessary for a specific sensory
perception to occur. It is known that neurons that express the same
complement of ligand-specific receptors send axons to a single olfactory
glomerulus (Vosshall & Stocker, 2007).
Hence, it is generally thought that glomerulus is an ideal location to
study sensory perception (Wang et al., 2003;
Semmelhack & Wang, 2009).
Since more than one sensory
neuron (olfactory neuron) is needed for perception to occur, it is
reasonable to assume about the presence of an interactive change
occurring between these neurons or their immediate output neurons. This
matches with the previous explanation for the generation of first-person
property of perception by the semblance hypothesis (Fig.1;
Vadakkan, 2015).
Based on the semblance
hypothesis, units of internal sensation of perception namely perceptons
are generated at the locations where two sensory inputs converge (Vadakkan,
2015). But the question is how does the fly recognize human odor
for the first time as something beneficial? Generation of the
first-person property of internal sensation concurrent with motor
actions to fly towards humans during first instance is most likely to
occur automatically by virtue of an inherited wiring mechanism. Hence,
the first instance of flight towards a human most likely occurs as
expected from an automaton. This and future events of flights towards
humans can lead to associations between sensory inputs taste of the
blood or filling of stomach or satiety can lead to formation of IPLs
that can generate both internal sensations necessary for survival
concurrent with appropriate motor actions. Hence, during later times,
this becomes a learned behavior.
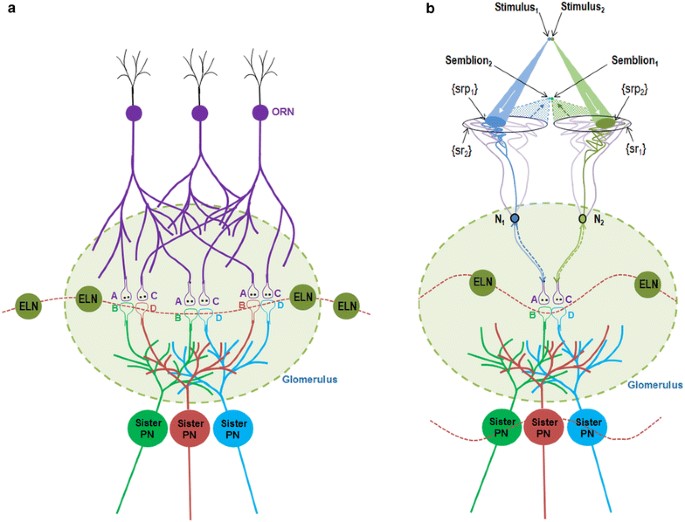
Figure 1.
Schematic diagram showing the mechanism of olfactory percept formation
within a glomerulus. a) Spread of activity through the neuronal
processes in the absence of odorants. The baseline firing of the
olfactory receptor neurons (ORNs) leads to spread of activity to the
synapses between the ORNs and the projection neurons (PNs). Spread of
activity through the excitatory local neurons (ELNs) from one glomerulus
to other glomeruli results in oscillating activity across different
glomeruli in the antennal lobe. Two postsynaptic terminals each from the
corresponding three different sister PNs whose dendrites are located
within a single glomerulus are shown. Based on the present work,
existing inter-postsynaptic LINKs within each of the different glomeruli
can contribute to horizontal component that can trigger oscillations of
potentials among the glomeruli. The integral of all the non-specific
semblances induced at the inter-postsynaptic LINKs is called C-semblance
that can contribute to the attention of the fly. A and C are the
presynaptic terminals of the ORNs. B and D are the postsynaptic
terminals (dendritic spines) of two different PNs within a glomerulus.
b) Induction of perceptons in the presence of an odorant. Two
synapses between two ORNs and two sister PNs within the glomerulus along
with their interpostsynaptic LINK (IPL) B–D is shown. In the context of
background C-semblance, the stimulus-semblion U-loops form at the
inter-postsynaptic LINK B–D to induce perceptons. Note that the
semblions are shown to overlap closer to the olfactory receptors than
the actual source of the odorant. This enables localization of the odor
close to the olfactory receptors, in contrast to the visual perception.
The entanglement of perceptons provides the conformation for the percept
of a specific smell. Percept of a specific attractive smell formed
within a glomerulus can trigger motor actions to the fly along the
concentration gradient as a response to increasing percepts, the fly can
reach towards the source of food. Note that the oscillating potential
wave form that extend beyond the single glomerulus in the absence of
odorants gets limited to that glomerulus alone due to the spread of
inhibitory activity to the other glomeruli through the inhibitory local
neurons (ILNs) during perception (Figure from Vadakkan, 2015).
Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB (2007) Two
chemosensory receptors together mediate carbon dioxide detection in
Drosophila. Nature 445(7123):86-90.
Semmelhack JL &
Wang JW (2009) Select Drosophila glomeruli mediate innate olfactory
attraction and aversion. Nature 459:218-223.
Vadakkan KI (2015)
A framework for the first-person internal sensation of visual perception
in mammals and a comparable circuitry for olfactory perception in
Drosophila. Springerplus 4:833.
Vosshall LB. &
Stocker RF (2007) Molecular architecture of smell and taste in.
Drosophila. Annu. Rev. Neurosci. 30:505-533.
Wang JW, Wong AM,
Flores J, Vosshall LB, Axel R (2003) Two-photon calcium imaging reveals
an odor-evoked map of activity in the fly brain. Cell
112:271-282.
Spine enlargement
following associative learning
Dopamine reduce excitatory postsynaptic currents (EPSCs) generated by
paraventricular thalamus (PVT) inputs to NAc, when carried out by whole
cell recording from medium spiny neurons (MSNs) of NAc
(Christoffel et al., 2021). This naturally leads to
the question, “What mechanistic explanation can satisfy the inference
that dopamine filter excitatory inputs to NAc?”
Based on IPL mechanism, formation of IPLs between dendritic spines of
MSNs that synapse with excitatory inputs from PVT neurons and dendritic
spines of MSNs that synapse with inhibitory inputs from ventral
tegmental area (VTA) takes place when dopaminergic inputs from VTA cause
expansion of spines of MSNs that synapse with excitatory inputs
(Vadakkan, 2019). The net effect will provide results equivalent to
filtering of excitatory inputs to NAc by dopamine.
Christoffel DJ, Walsh JJ, Hoerbelt P, Heifets
BD, Llorach P, Lopez RC, Ramakrishnan C, Deisseroth K,
Malenka RC
(2021)
Selective filtering of excitatory inputs to nucleus accumbens by
dopamine and serotonin.
Proc
Natl Acad Sci U S A.118(24):e2106648118.
PubMed
Vadakkan K.I (2019) Internal sensation of pleasure can be explained as a specific conformation of semblance: Inference from electrophysiological findings. Peerj Preprints Article
Drift in the set of neurons in
the primary olfactory cortex that fire in response to an odour
Several studies have observed correlation
between odorants and specific sets of neurons that fire in response to
them. Continuous recording from these neurons show that this correlation
is lost after several weeks (Schoonover et al., 2021). (Schoonover et
al., 2021). Authors suspected that this instability reflects the
unstructured connectivity of piriform cortex. What property of the
circuitry will cause such a drift? It further leads to more fundamental
questions such as “What is a percept?” “Where is it formed?”
It
was possible to explain a framework of a mechanism of perception based
on the IPL mechanism (Vadakkan, 2011). During associative learning
events, new IPL are formed in the cortices. Even though olfactory
stimuli propagate directly to the hippocampus without propagating to an
intermediate association cortex (Zhou et al., 2021), outputs from the
hippocampus can generate IPLs in the cortex. Insertion of new neurons in
the pathways (in the granule layer of hippocampus) through which signals
from associatively learned items/events propagate, along with exposure
of the system to new associative learning items/events that share
elements of the previously associated items/events, will lead to
continuous formation of new IPLs in the cortices (Vadakkan, 2010; 2016).
This will lead to changes in the summated potentials arriving to the
neurons in the olfactory cortex. Hence, firing property of neurons in
the primary olfactory cortex during perception of the same stimulus is
expected to show continuous drift.
Based on the semblance
hypothesis, when perception is viewed as first-person internal
sensations, it was possible to find a framework of a mechanism for
perception (Vadakkan, 2015). Accordingly, internal sensation of a
percept is formed by integral of all perceptons, unitary mechanisms of
perception. Large number of redundant perceptons are expected to form.
Hence, the net integral of all perceptons remain almost same, even with
changes in the locations from where perceptons are formed. Furthermore,
extreme degeneracy of attenuating input signals in firing a neuron
(Vadakkan, 2019) indicates that perceptons are generated at the input
level. Correlations with neuronal firing will only be true for those
neurons that are being held at sub-threshold activation state and
receive additional potentials through inter-postsynaptic functional
LINKs (IPLs) at the time of perception. Hence, internal sensation of
perception continues to take place even when the set of neurons that
fires changes over time due to changes in the circuitry.
Schoonover CE, Ohashi SN, Axel R,
Fink AJP
(2021)
Representational drift in primary olfactory cortex.
Nature. 2021 594(7864):541-546.
Zhou G, Olofsson JK, Koubeissi MZ, Menelaou
G, Rosenow J, Schuele SU, Xu P, Voss JL, Lane G,
Zelano C
(2021) Human hippocampal connectivity
is stronger in olfaction than other sensory systems.
Prog Neurobiol. 201:102027.
Vadakkan KI (2011)
A possible mechanism of transfer of memories from the hippocampus to the
cortex.
Med Hypotheses. 77(2):234-43.
A framework for the first-person internal sensation of visual perception
in mammals and a comparable circuitry for olfactory perception in
Drosophila.
Springerplus. 4:833.
PubMed
Vadakkan KI (2016)
The functional role of all postsynaptic potentials examined from a
first-person frame of reference. Rev Neurosci. 27(2):159-84.
Vadakkan KI (2019) Extreme degeneracy of
inputs in firing a neuron leads to loss of information when neuronal
firing is examined.
Peerj Preprints. Article
Pathological features of Alzheimer's disease such as tangles & plaques start appearing in normally aging brains (Was able to explain this recently: Aging as a loss of an adaptation that stabilizes last developmental stage of the nervous system)
Examination of IPLs derived by semblance
hypothesis has led to the inference that the last stage of its development
undergoes an adaptation whereby inter-neuronal inter-spine fusion
is prevented by arresting it at/before the stage of hemifusion
(Vadakkan, 2020). This is based on the following observations. In the
mouse, neuronal precursor cells in the ventricular zone (VZ) undergo
cell division.
While in the VZ,
100% of precursors in G2 and S phases of the cell cycle couple
together and form clusters (Bittman et al., 1997). During this stage,
injection of dye into one cell spread to neighouring cells (Bittman et
al., 1997). This indicates formation of fusion pores between these
cells. This is followed by
death of nearly
70% of these cells and survival of the remaining 30% cells (Blaschke et
al., 1996). The surviving 30% of cells are expected to have acquired an
adaptation most probably during inter-cellular coupling. The adaptation
most likely prevents any future coupling between neurons that may result
in inter-neuronal fusion. This adaptation is suitable for maintaining IPLs
(that generated inner sensations) and prevents any IPL fusion. Aging can be viewed as resulting from gradual loss of this
adaptation. Augmented formation IPL fusion events can lead to
pathological changes such as those observed in neurodegenerative
disorders (Vadakkan, 2019). For example, pathological changes of
neurofibrillary tangles and amyloid plaques can result from
precipitation of proteins & leakages of certain precipitated proteins
through defective fusion pores to the extracellular matrix space in Alzheimer’s disease.
If semblance hypothesis is
correct, then its corollary that these pathological findings should also be
found in normal aging can be verified. Since senile
neurofibrillary tangles and amyloid plaques appear in normally aging brains (Anderson, 1997;
),
this forms sufficient verification. This reinforces the need for testing
the predictions of semblance hypothesis.
Vadakkan KI (2020) A derived mechanism of nervous system functions
explains aging-related neurodegeneration as a gradual loss of an
evolutionary adaptation. Curr Aging Sci 13(2):136–152.
Bittman K, Owens DF, Kriegstein AR, LoTurco JJ (1997) Cell coupling and uncoupling in the ventricular zone of developing neocortex. Journal of Neuroscience 17(18):7037-7044. PubMed
Vadakkan KI (2016) Neurodegenerative disorders share common features of
"loss of function" states of a proposed mechanism of nervous system
functions. Biomed Pharmacother. 83:412-430.
Anderton BH (1997) Changes in the ageing brain in health and disease.
Philos Trans R Soc Lond B Biol Sci. 352(1363):1781-1792.
Heterogeneity of neurons in the cortex
Studies of cortical neurons show significant heterogeneity in transcriptomic analyses (; Tasic et al., 2018; Hodge et al., 2019). In fact, these findings show that there won't be two neurons with same sets of transcripts within them. The above findings naturally raise the question, "What is the functional importance of such a finding?" The actual operational mechanism of the nervous system is expected to provide clues for a suitable explanation. Based on the IPL mechanism, this heterogeneity is necessary for the formation of IPL fusion between spines that belong to different neurons at one stage of development supported by the diffusion of dye injected into on neuron to neighboring neurons (see, Vadakkan, 2020). If neurons are not heterogeneous, then fusion between them will not evoke cellular reactions, which is responsible for cell death of majority of neurons. Most importantly, this IPL fusion is expected to trigger an adaptation in surviving neurons, responsible for restricting IPL fusion to the stage of IPL hemifusion. Thus, neuronal heterogeneity can be viewed as a marker of an adaptation that occurred during that last stages of the developmental of the nervous system. It is most likely that maintaining heterogeneity is essential for maintaining the above adaptation throughout the life-span of the neurons. This prompts to make a testable prediction that, any deficiencies in maintaining this adaptation will trigger IPL fusion between heterogeneous neurons, which can explain aging and other disease associated neurodegeneration.
Tasic et al., (2016) Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci. 19(2):335-346. PubMed
Spatial gene-expression gradients underlie prominent heterogeneity of CA1 pyramidal neurons. Neuron. 89(2):351-68. PubMed
Tasic et al., (2018) Shared and distinct transcriptomic cell types
across neocortical areas. Nature 2018 563 (7729):72-78
Hodge et al., (2019) Conserved cell types with divergent features in
human versus mouse cortex. Nature 573 (7772):61-68
Spine depolarization without dendritic depolarization
It was found that in excitatory synapses, large spine depolarization recruit voltage-dependent channels without dendritic depolarization, due to high spine neck resistance (Beaulieu-Laroche and Harnett, 2018). Hence, it leads to the questions, "What is the functional importance of seemingly isolated spine depolarization?" and "Since this is a conserved property, how to provide a mechanistic explanation in terms of brain functions?" Another finding from the same laboratory is that distal human dendrites provide limited excitation to the soma even in the presence of dendritic spikes (Beaulieu-Laroche et al., 2018). The observation that even dendritic spikes have only a limited role in neuronal firing is of huge significance. This again reinforces the need for figuring out the functions achieved by depolarization of spine heads in excitatory cortical neurons. IPL mechanism can explain how depolarization of spines is associated with generation of units of internal sensations independent of neuronal firing. These experimental findings compel us to undertake dedicated experimental verification of the IPL mechanism.
Beaulieu-Laroche L and Harnett MT. 2018. Dendritic spines prevent synaptic voltage clamp. Neuron 97(1): 75–82.e3. PubMed
Beaulieu-Laroche L, Toloza EHS, van der Goes MS, Lafourcade M, Barnagian D, Williams ZM, Eskandar EN, Frosch MP, Cash SS, Harnett MT. 2018. Enhanced dendritic compartmentalization in human cortical neurons. Cell 175(3): 643–651.e14. PubMed
Largest class of neurons in the visual cortex is not reliably responsive
to any of the visual stimuli
In a recent report by de Vries et al.,
(2020), the authors examined firing of nearly 60,000 visual cortical
neurons in response to different visual stimuli. They found that while
most classes of these neurons respond to specific subsets of stimuli,
the largest class is not reliably responsive to any of the stimuli. The
latter
finding supports
the observations made by semblance hypothesis during visual perception
(Vadakkan, 2016). Accordingly, the internal sensation of perception
takes place at the inter-LINKed spines and is independent of firing of
their neurons. Moreover, postsynaptic potentials generated by visual
stimuli at these inter-LINKed spines need not necessarily add potentials
to raise the summated potentials to reach the threshold level
for firing those neurons (Vadakkan, 2019). Therefore, as per semblance hypothesis, the
expectation is that a huge set of neurons will not be responsive to any
visual sensory stimuli even when internal sensation of vision takes
place. The report by De Vries et al., (2020) is in agreement with the
expectations of the mechanism of visual perception provided by semblance hypothesis.
Their finding that most
classes of visual cortical neurons respond to specific subsets of
stimuli indicates that the propagation of stimuli to higher cortical
areas is necessary for performing secondary functions such as a) “where”
and “what” associative properties of visual stimuli at higher cortical
areas, and b) associative learning with other sensory stimuli at
different associative cortical areas.
de Vries et al.,
(2020)
A large-scale standardized physiological survey reveals functional
organization of the mouse visual cortex.
Nat Neurosci.
2020 Jan;23(1):138-151. doi: 10.1038/s41593-019-0550-9.
PubMed
Vadakkan KI
(2016)
A framework for the first-person internal sensation of visual perception
in mammals and a comparable circuitry for olfactory perception in
Drosophila.
Springerplus.
2015 Dec 30;4:833. doi: 10.1186/s40064-015-1568-4. eCollection 2015.
PubMed
Artificial firing of a neuron leads to firing of a set of neurons of the same neuronal order
In a recent work
by Chettih and Harvey (2019), authors artificially triggered several
spikes (action potentials) in single neurons in layer 2/3 of mouse
visual cortex V1area. This resulted in spiking activity in a group of
sparsely distributed neighbouring neurons in the same neuronal order and
were correlated in time.
The small population of neurons that were excited were located at
short distance (25–70µm) from the stimulated neuron. The stimulation had no influence beyond 300µm
(for a summary, see News and Views article by Ikuko Smith (Smith, 2019).
The authors
called this lateral spread of activity between neurons
"influence-mapping."
There is one important question. How does
excitation reach at the laterally located neurons in a time-correlated
manner, which is responsible for influence-mapping? This can be
explained by the testable mechanism derived by semblance hypothesis (Fig.1). It
is related to the previous explanation of visual perception as a
first-person property using the derived mechanism of generation of
internal sensation at physiological time-scales (Vadakkan, 2016). The
units of internal sensation of perception are induced at the
inter-LINKed spines that belong to different neurons. When a single
neuron is artificially fired, the back propagating action potentials
will reach the dendritic spines. It will then continue to propagate
through the inter-LINKed spines to the neuronal soma of the inter-LINKed
spine’s neuron (Fig. 2). The spines that inter-LINK can belong to
neurons that are separated by up to 300µm, a distance beyond which the
probability of overlapping of dendritic arbor between neurons diminishes
substantially.
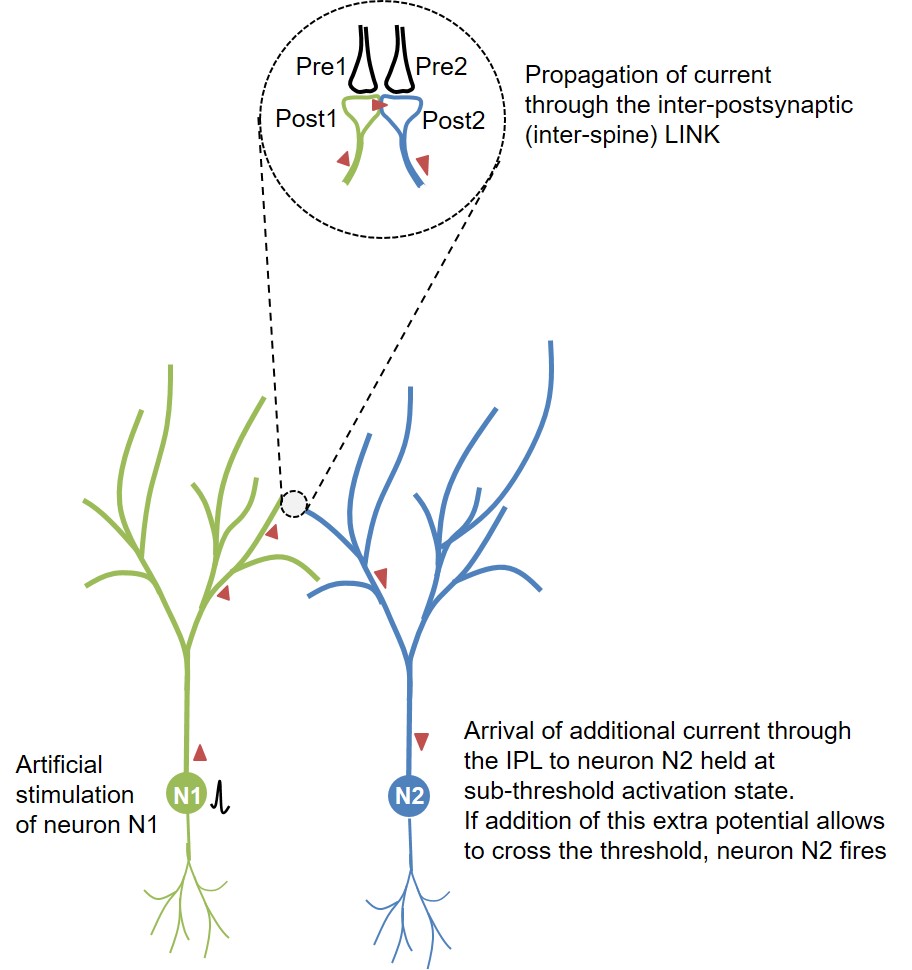
Figure 1. Schematic diagram
showing the route of propagation of action potential from the
artificially fired neuron N1 towards the sparsely located neuron N2
within the layer2/3 in visual cortex. This spread taking place through
the inter-LINKed spines Post1 and Post2 can explain what the authors
describe as “influence-mapping.” Note that the inter-postsynaptic
functional LINK (IPL) between Post1 and Post2 was explained as
responsible of induction of internal sensation for perception (Vadakkan,
2015). Overlapping of the dendritic arbors between the neurons N1 and N2
increases the probability of IPL formation when neurons N1 and N2 are
separated only by a short distance (25–70µm).
b) When a neuron was fired, the majority of
neurons that were tuned to respond to similar features to that neuron
were strongly suppressed than the neurons with a different tuning
regardless of the distance from the stimulated neuron. Inhibition of the
spikes in the neighbouring neurons can be explained by activation of
surrounding inhibitory interneurons. Burst of action potentials in
excitatory neurons can activate somatostatin expressing inhibitory
interneurons (Kwan and Dan 2012). Similar type of inhibition of
surrounding areas is seen in locations where the internal sensation of
perception is expected to occur in the olfactory glomeruli in Drosophila. When one glomerulus is activated, inhibitory local
interneurons (ILN) inhibit all the remaining glomeruli (Hong and Wilson
2015) enabling the specificity of the percept for that particular smell
(Vadakkan, 2015).
Orientation tuning is tested by a source of
light. This will cause activation of a large number of islets of
inter-LINKed spines within one cortical column. But when single neurons
are artificially fired the backpropagation of potentials will reach only
specific sets of inter-LINKed spines. This explains why only neurons
that are located sparsely are fired, correlated in time.
Verification: Based on semblance hypothesis,
the prediction that can be made is the presence of inter-postsynaptic
functional LINKs (IPLs) between spines that belong to the artificially
fired neuron and the sparsely located neurons that were fired in a
time-correlated manner.
Chettih SN, Harvey CD (2019) Single-neuron
perturbations reveal feature-specific competition in V1. Nature doi:
10.1038/s41586-019-0997-6.
PubMed
Smith IT (2019) The influence of a single neuron on its network. Nature. 567(7748):320-321 PubMed
Kwan AC, Dan Y (2012) Dissection of
cortical microcircuits by single-neuron stimulation in vivo. Current
Biology 22, 1459–1467.
PubMed
Vadakkan KI (2015) A framework for the
first-person internal sensation of visual perception in mammals and a
comparable circuitry for olfactory perception in Drosophila.
Springerplus 4:833.
PubMed
Hong EJ, Wilson RI (2015) Simultaneous
encoding of odors by channels with diverse sensitivity to inhibition.
Neuron 85(3):573–589.
PubMed
Memory retrieval occurs at a frequency of oscillating extracellular potentials similar to that was present during learning
A recent study examined the nature of oscillating extracellular
potential both during learning and memory retrieval (Vaz et al.. 2019).
In order to reactivate the same set of IPLs
that formed during learning at the time of memory retrieval, it is
necessary to have almost similar conditions that were present at the
time of learning. Maintaining the same frequency of oscillating
extracellular potentials is a major factor in achieving this. Based on
the semblance hypothesis, the synaptic transmission in one direction and
propagation of potentials in a near-perpendicular direction through the
inter-postsynaptic functional LINK (IPL) contribute vector components to
the oscillating extracellular potentials, which is essential for binding
and integration of units of internal sensations for providing the
sensory qualia of memory. The findings of this study that show that
similar frequency of oscillating extracellular potentials are present
both during learning and memory retrieval support the expectations of
semblance hypothesis.
Vaz AP, Inati SK, Brunel N, Zaghloul KA (2019) Coupled ripple oscillations between the medial temporal lobe and neocortex retrieve human memory. Science. 363:975-978. PubMed
Dendritic calcium spikes that are related to behavior and cognitive function
Similar to the action potentials (axonal spikes or neuronal firing) occurring at the axonal hillock, there are spikes occurring at the dendrites. These are called dendritic spikes. Based on the strength of summated potentials, a rough estimate shows that they constitute synchronous activation of nearly 10 to 50 neighboring glutamatergic synapses triggering a local regenerative potential (Antic et al., 2010). Depending on the channels involved, there are different types of dendritic spikes. Recently, it was found that distal dendrites generate dendritic spikes whose firing rate is nearly five times greater than at the cell body (Moore et al., 2017). Another group of investigators who have previously shown that dendritic spikes are related to behavior and cognitive function recently found that dendritic calcium spikes contribute to surface potentials that are recorded as electroencephalogram (EEG) (Suzuki et al., 2017). Surface EEG recording is generated by current sink that reflects the net potential changes within the extracellular matrix space. This is expected to be contributed by several factors. It is known that the surface positive potentials are generated mainly by synaptic inputs from other cortical and subcortical regions to the pyramidal neurons located between L2/3 to L4 regions (Douglas and Martin, 2004). Recent studies by Suzuki et al., has found that dendritic calcium spikes at the main bifurcation points of the apical dendrites of L5 pyramidal neurons (note that L5 pyramidal neurons are upper motor neurons that direct motor movement of the body) also generate the surface positive potentials (Suzuki et al., 2017).
The last two findings lead to the questions, “How can two different sources of potentials provide similar surface positive potentials?" "Can we provide an interconnected explanation?" Since dendritic spikes are related to both behavior and cognitive functions and since IPL mechanism can explain generation of concurrent internal sensation of memory and behavioral motor action, can IPL mechanism explain the above findings? Since the apical tuft regions of all the pyramidal neurons are anchored to the pial surface, the dendritic arbor of all the pyramidal neurons is overlapped at the recording location of Suzuki et al., (2017). In this context, it is necessary to examine the potential changes occurring at the neuronal processes around the recording electrode. In the context of the IPL mechanism, it is anticipated that the dendritic spines of different neurons have formed a large number of islets of IPLs between them at these locations. By examining the zone from where low-threshold calcium spikes were recorded (Suzuki et al., 2017; Larkum and Zhu, 2002), the following is possible.
Spatially, main bifurcation points of the apical dendrites of L5 pyramidal neurons are also locations where spines of the L2/3 pyramidal neurons receive their input. Based on the IPL mechanism, several of these spines are expected to be inter-LINKed to form large islets. These islets are also expected to be inter-LINKed with spines of L5 pyramidal neurons for initiating or controlling motor actions. The potentials through the IPLs are expected to arrive at the axon hillock of the L5 motor neurons that are kept at a sub-threshold state (see figure 5 in the FAQ section of this website) for the motor action (Fig.2). For a system that operates to generate internal sensations and initiates or controls concurrent motor actions, the islets at appropriate locations are expected to transmit potentials to the axon hillock of the L5 pyramidal neurons that are upper motor neurons. Calcium spikes are generated at the postsynaptic locations within the islet of inter-LINKed spines possibly due to an increased density of these channels at these locations. Since the pyramidal neurons are found to be under the influence of an inhibitory blanket (Karnani et al., 2014), a function of dendritic spikes is to generate sufficient potentials to overcome this inhibition. In other words, there is a provision for increasing the inhibitory blanket around an L5 pyramidal neuron axon hillock as the size of the islets of inter-LINKed spines that are connected to these neurons increases. This will make sure that the L5 neuron fires only at the activation of specific sets of IPLs that generates a specific conformation of semblance for both the internal sensation and concurrent behavioral motor action.
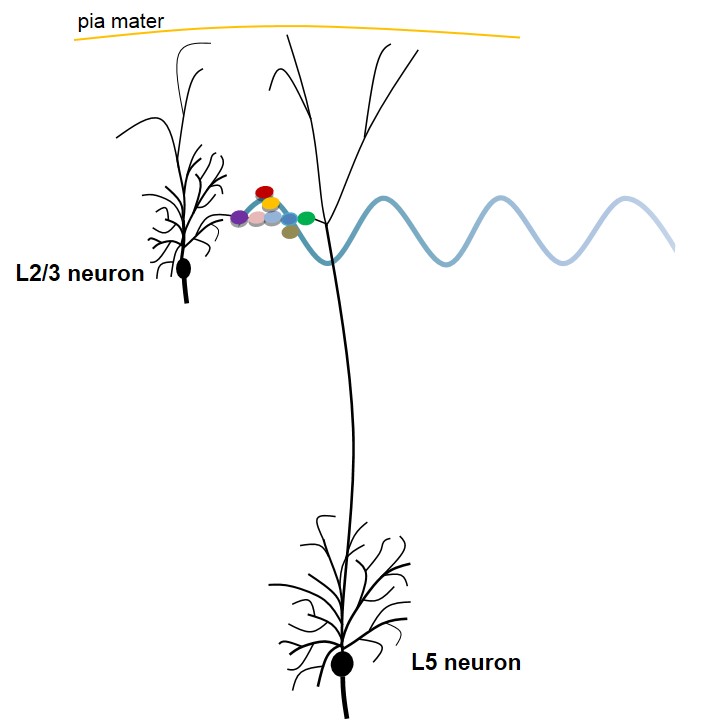
Figure 2. Figure explaining a potential mechanism occurring at the level of the main bifurcation point of an apical dendrite of an L5 pyramidal neuron (based on semblance hypothesis). The circles with different colors represent an islet of inter-LINKed spines (dendritic spines or postsynaptic terminals) that belong to different pyramidal neurons at the level of the main bifurcation point of the apical dendrite of L5 neuron. Note that one of the spines (in violet) belongs to one of the L2/3 pyramidal neurons. Also note that the inter-LINKed spine on the far right end of the islet (in green) belongs to L5 pyramidal neuron. During development, neurons of different cortical neuronal orders descend from the inner pial surface area by anchoring the apical dendritic terminals to the inner pial region. This allows overlapping of the dendritic arbors of neurons from different orders, which leads to abutting of their spines that eventually leads to the formation of inter-LINKs between these spines during learning. The waveform shown at the level of the inter-LINKed spines indicates that the oscillating extracellular potentials recorded have a major contribution from the propagation of potentials through the islets of inter-LINKed spines. Secondary factors can determine different wave forms depending on the locations from where recording is carried out. They include a number of neuronal layers, recurrent collaterals, connections with the projection neurons from other ares of the brain, etc. Figure not to scale (spines in the islet are drawn disproportionately large compared to the size of neurons).
The explanation that synaptic transmission and propagation of potentials through the IPLs provide vector components of oscillating extracellular potentials also becomes suitable. If the arrival of potentials from sensory stimuli evokes dendritic calcium spikes along with the reactivation of specific inter-LINKed spines (and their islets) inducing units of specific internal sensations concurrent with activation of specific sets of motor neurons, it can provide an explanation how dendritic calcium spikes are related to behavior and cognitive function. The findings of Suzuki et al., necessitate examining the role of background EEG wave forms, frequency of which correlates with normal level of consciousness. In this regard, the explanation by the IPL mechanism that the net background semblance induced by reactivation of inter-LINKed spines contributes to the internal sensation of consciousness (Vadakkan, 2010) becomes a suitable mechanism that can be subjected to further studies.
Antic SD, Zhou WL, Moore AR, Short SM, Ikonomu KD (2010) The decade of the dendritic NMDA spike. J Neurosci Res. 88(14):2991–3001 PubMed
Moore JJ, Ravassard PM, Ho D, Acharya L, Kees
AL, Vuong C, Mehta MR (2017) Dynamics of cortical dendritic membrane
potential and spikes in freely behaving rats.
Science.
355(6331) PubMed
Suzuki M, Larkum ME (2017)
Dendritic calcium spikes are clearly detectable at the cortical surface.
Nat Commun. 8(1):276
Douglas RJ, Martin KA (2004) Neuronal circuits of
the neocortex.
Annu. Rev. Neurosci.
27: 419–451
Larkum ME, Zhu JJ (2002) Signaling of layer 1
and whisker-evoked Ca2+ and Na+ action potentials in distal and terminal
dendrites of rat neocortical pyramidal neurons in vitro and in vivo. J.
Neurosci. 22, 6991–7005
Regenerative spikes at the dendritic arbor - a mechanism for internal sense of a place that reflects binding at the time of learning
Each place field consists
of a unique set of CA1 neurons that fire action potential. At the
dendritic regions, calcium transients inform about a change in
potentials occurring regeneratively either due to back propagating
action potentials (bAP) or by dendritic spikes. Recent studies observed
calcium transients secondary to regenerative dendritic events in place
cells that can predict place field properties (Sheffield and Dombeck,
2015a; Sheffield et al., 2017). These calcium transients have a highly
spatiotemporally variable prevalence throughout the dendritic arbor. In
some cases only a subset of the observed branches displayed detectable
spikes, which indicates that spikes originated at these dendritic
branches. None of the observed branches in many cases displayed
detectable spikes during place field traversals while the soma (and
axon) fired. This means that the bAP did not reach these locations.
The above finding can be
explained by the occurrence of dendritic spike occurs at an islet of
inter-LINKed spines that belong to different CA1 neurons
(Vadakkan, 2013). This has the following advantages.
a) Activation of inter-LINKed spines within an islet of inter-LINKed
spines induces units of internal sensations for a specific place. b) One
dendritic spike at an islet of inter-LINKed spines that belong to
different neurons can explain the firing of different CA1 neurons that
are being maintained in a sub-threshold state at the time of the dendritic
spike. It also supports why a high percentage of place cells are shared
between different places. c) Since potentials degrade as they reach the
axonal hillock, it may require potentials arriving from more than one spike to contribute to the
firing of a CA1 neuron depending on latter’s sub-threshold level. d) The
highly
spatiotemporally variable nature of spike depends on the qualia of
internal sensations that they induce in response to and matching with
the place (which depends on previous associative learning events with
different places).
Sheffield MEJ, Dombeck DA (2015a) Calcium transient prevalence across the dendritic arbour predicts place field properties. Nature. 517(7533):200-204. PubMed
Sheffield MEJ, Adoff MD,
Dombeck DA (2017) Increased Prevalence of Calcium Transients across the
Dendritic Arbor during Place Field Formation. Neuron. 96(2):490-504.e5
PubMed
Vadakkan KI (2013) A supplementary circuit rule-set for neuronal wiring. Frontiers in Human Neuroscience. 7:170 PubMed
Sheffield ME, Dombeck DA (2015b) The binding solution? Nature Neuroscience. 18(8):1060-102 PubMed
B. In pathological conditions
Spread of epileptic activity
Epileptic activity in the
hippocampus propagates with or without synaptic transmission at a
speed of nearly 0.1m/s (Jefferys, 2014). Experiments showed that the longitudinal
propagation of epileptic activity from one end of a neuronal order to
its other end in the hippocampus takes
place independent of chemical or electrical synaptic transmission (Zhang
et al., 2014). Since this spread of epileptic activity occurs at a speed
of 0.1 m/s and is not compatible with ionic diffusion or pure axonal
conduction (Jefferys 2014; Zhang et al., 2014), it requires an
explanation at the cellular and electrophysiological levels. In this regard, rapid chain
propagation through the inter-postsynaptic functional LINKs (IPLs) explained by the semblance hypothesis (Vadakkan, 2015) offers
a suitable explanation for a mechanism.
Jefferys JG
(2014)
How does epileptic activity spread?
Epilepsy Currents. 14(5):289-290
PubMed
Zhang M, Ladas TP, Qiu C, Shivacharan RS,
Gonzalez-Reyes LE, Durand DM (2014) Propagation of epileptiform activity
can be independent of synaptic transmission, gap junctions, or diffusion
and is consistent with electrical field transmission. Journal of
Neuroscience. 2014 34(4):1409-1419
PubMed
Vadakkan KI (2016) Rapid chain generation
of interpostsynaptic functional LINKs can trigger seizure generation:
Evidence for potential interconnections from pathology to behavior.
Epilepsy & Behavior. 59:28-41
PubMed
Heterogeneity of clinical and pathological findings in Alzheimer's disease
Alzheimer's disease (and most other neurodegenerative disorders) are
highly heterogeneous in its clinical and pathological features (Lam et
al., 2013;
Tasic et al., (2016) Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci. 19(2):335-346. PubMed
Spatial gene-expression gradients underlie prominent heterogeneity of CA1 pyramidal neurons. Neuron. 89(2):351-68. PubMed
Tasic et al., (2018) Shared and distinct transcriptomic cell types
across neocortical areas. Nature 2018 563 (7729):72-78
Hodge et al., (2019) Conserved cell types with divergent features in
human versus mouse cortex. Nature 573 (7772):61-68